Abstract
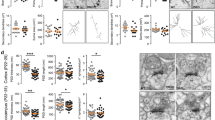
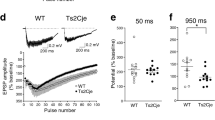
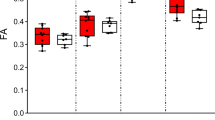
ZBTB18/RP58 (OMIM *608433) is one of the pivotal genes responsible for 1q43q44 microdeletion syndrome (OMIM #612337) and its haploinsufficiency induces intellectual disability. However, the underlying pathological mechanism of ZBTB18/RP58 haploinsufficiency is unknown. In this study, we generated ZBTB18/RP58 heterozygous mice and found that these mutant mice exhibit multiple behavioral deficits, including impairment in motor learning, working memory, and memory flexibility, which are related to behaviors in people with intellectual disabilities, and show no gross abnormalities in their cytoarchitectures but dysplasia of the corpus callosum, which has been reported in certain population of patients with ZBTB18 haploinsufficiency as well as in those with 1q43q44 microdeletion syndrome, indicating that these mutant mice are a novel model of ZBTB18/RP58 haploinsufficiency, which reflects heterozygotic ZBTB18 missense, truncating variants and some phenotypes of 1q43q44 microdeletion syndrome based on ZBTB18/RP58 haploinsufficiency. Furthermore, these mice show glutamatergic synaptic dysfunctions, including a reduced glutamate receptor expression, altered properties of NMDA receptor-mediated synaptic responses, a decreased saturation level of long-term potentiation of excitatory synaptic transmission, and distinct morphological characteristics of the thick-type spines. Therefore, these results suggest that ZBTB18/RP58 haploinsufficiency leads to impaired excitatory synaptic maturation, which in turn results in cognitive dysfunction in ZBTB18 haploinsufficiency.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Maulik PK, Mascarenhas MN, Mathers CD, Dua T, Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32:419–36.
Tunnicliffe P, Oliver C. Phenotype-environment interactions in genetic syndromes associated with severe or profound intellectual disability. Res Dev Disabil. 2011;32:404–18.
Ellison JW, Rosenfeld JA, Shaffer LG. Genetic basis of intellectual disability. Annu Rev Med. 2013;64:441–50.
Depienne C, Nava C, Keren B, Heide S, Rastetter A, Passemard S, et al. Genetic and phenotypic dissection of 1q43q44 microdeletion syndrome and neurodevelopmental phenotypes associated with mutations in ZBTB18 and HNRNPU. Hum Genet. 2017;136:463–79.
Tung Y, Lu H, Lin W, Huang T, Kim S, Hu G, et al. Case report: identification of a de novo microdeletion 1q44 in a patient with seizures and developmental delay. Front Genet. 2021;12:1–7.
Selmer KK, Bryne E, Rødningen OK, Fannemel M. A de novo 163 kb interstitial 1q44 microdeletion in a boy with thin corpus callosum, psychomotor delay and seizures. Eur J Med Genet. 2012;55:715–8.
Ballif BC, Rosenfeld JA, Traylor R, Theisen A, Bader PI, Ladda RL, et al. High-resolution array CGH defines critical regions and candidate genes for microcephaly, abnormalities of the corpus callosum, and seizure phenotypes in patients with microdeletions of 1q43q44. Hum Genet. 2012;131:145–56.
McRae JF, Clayton S, Fitzgerald TW, Kaplanis J, Prigmore E, Rajan D, et al. Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–8.
Aoki K, Meng G, Suzuki K, Takashi T, Kameoka Y, Nakahara K, et al. RP58 associates with condensed chromatin and mediates a sequence- specific transcriptional repression. J Biol Chem. 1998;273:26698–704.
Okado H. Regulation of brain development and brain function by the transcriptional repressor RP58. Brain Res. 2019;1705:15–23.
Okado H. Nervous system regulated by POZ domain Krüppel-like zinc finger (POK) family transcription repressor RP58. Br J Pharm. 2021;178:813–26.
Ohtaka-Maruyama C, Miwa A, Kawano H, Kasai M, Okado H. Spatial and temporal expression of RP58, a novel zinc finger transcriptional repressor, in mouse brain. J Comp Neurol. 2007;502:1098–108.
Okado H, Ohtaka-Maruyama C, Sugitani Y, Fukuda Y, Ishida R, Hirai S, et al. The transcriptional repressor RP58 is crucial for cell-division patterning and neuronal survival in the developing cortex. Dev Biol. 2009;331:140–51.
Ohtaka-Maruyama C, Hirai S, Miwa A, Heng JIT, Shitara H, Ishii R, et al. RP58 regulates the multipolar-bipolar transition of newborn neurons in the developing cerebral cortex. Cell Rep. 2013;3:458–71.
Hirai S, Miwa A, Ohtaka-Maruyama C, Kasai M, Okabe S, Hata Y, et al. RP58 controls neuron and astrocyte differentiation by downregulating the expression of Id1-4 genes in the developing cortex. EMBO J. 2012;31:1190–202.
Fujihara K, Miwa H, Kakizaki T, Kaneko R, Mikuni M, Tanahira C, et al. Glutamate decarboxylase 67 deficiency in a subset of GABAergic neurons induces schizophrenia-related phenotypes. Neuropsychopharmacology 2015;40:2475–86.
Cohen JS, Srivastava S, Farwell Hagman KD, Shinde DN, Huether R, Darcy D, et al. Further evidence that de novo missense and truncating variants in ZBTB18 cause intellectual disability with variable features. Clin Genet. 2017;91:697–707.
Cargnin F, Kwon JS, Katzman S, Chen B, Lee JW, Lee SK. FOXG1 orchestrates neocortical organization and cortico-cortical connections. Neuron. 2018;100:1083–96.e5.
Hering H, Sheng M. Dendritic spines: structure, function and regulation. Nat Rev Neurosci. 2001;2:880–8.
Pchitskaya E, Bezprozvanny I. Dendritic spines shape analysis—classification or clusterization? Perspective. Front Synaptic Neurosci. 2020;12:31.
Rauch A, Wieczorek D, Graf E, Wieland T, Endele S, Schwarzmayr T, et al. Range of genetic mutations associated with severe non-syndromic sporadic intellectual disability: an exome sequencing study. Lancet. 2012;380:1674–82.
De Munnik SA, García-Miñaúr S, Hoischen A, Van Bon BW, Boycott KM, Schoots J, et al. A de novo non-sense mutation in ZBTB18 in a patient with features of the 1q43q44 microdeletion syndrome. Eur J Hum Genet. 2014;22:844–6.
Uddin LQ. Cognitive and behavioural flexibility: neural mechanisms and clinical considerations. Nat Rev Neurosci. 2021;22:167–79.
Balschun D, Moechars D, Callaerts-Vegh Z, Vermaercke B, Van Acker N, Andries L, et al. Vesicular glutamate transporter VGLUT1 has a role in hippocampal long-term potentiation and spatial reversal learning. Cereb Cortex. 2010;20:684–93.
Choi DW. Calcium-mediated neurotoxicity: relationship to specific channel types and role in ischemic damage. Trends Neurosci. 1988;11:465–9.
McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Rev. 1990;15:41–70.
Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56.
O’Donnel C, Nolan MF, van Rossum MCW. Dendritic spine dynamics regulate the long-term stability of synaptic plasticity. J Neurosci. 2011;31:16142–56.
van der Schoot V, de Munnik S, Venselaar H, Elting M, Mancini GMS, Ravenswaaij-Arts CMA, et al. Toward clinical and molecular understanding of pathogenic variants in the ZBTB18 gene. Mol Genet Genom Med. 2018;6:393–400.
Wang T, Hoekzema K, Vecchio D, Wu H, Sulovari A, Coe BP, et al. Large-scale targeted sequencing identifies risk genes for neurodevelopmental disorders. Nat Commun. 2020;11:1–13.
Acknowledgements
We thank Dr. Masataka Kasai for the use of RP58 mutant mice, Dr. Minoru Saitoe for their helpful comments on the manuscript, Dr. Yoko Tsukamoto for the initial electrophysiological experiment, Dr. Shigeo Okabe for the valuable suggestions on the morphological analysis of the spines, and the members of our laboratory for their technical assistance and critical comments. This work was supported by Grants-in-Aid for Scientific Research (KAKENHI) from the Ministry of Education, Science, Sports, Culture and Technology of Japan (19K08033, 18KK0442), and Intramural Research Grant (3-1) for Neurological and Psychiatric Disorders provided by the National Center of Neurology and Psychiatry, Japan (to HM), and KAKENHI (18H02537, 18K19383, 22H02729) (to HO).
Author information
Authors and Affiliations
Contributions
Sayaka H (SaH), HM, HS, and HO. designed the research; SaH, HM, HS, KN, MK, TT, COM, Shinobu H (Sh H), and HO performed the research; SaH, HM, HS, KN, MK, TT, COM, ShH, and HO analyzed the data; SaH, HM, HS, ShH and HO prepared the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hirai, S., Miwa, H., Shimbo, H. et al. The mouse model of intellectual disability by ZBTB18/RP58 haploinsufficiency shows cognitive dysfunction with synaptic impairment. Mol Psychiatry 28, 2370–2381 (2023). https://doi.org/10.1038/s41380-023-01941-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-023-01941-3