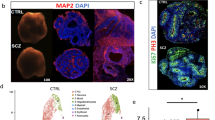
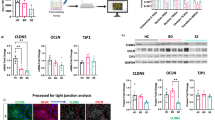
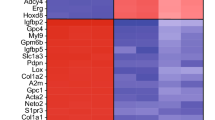
Abstract
Schizophrenia (SZ) is a complex neuropsychiatric disorder, affecting 1% of the world population. Long-standing clinical observations and molecular data have pointed to a possible vascular deficiency that could be acting synergistically with neuronal dysfunction in SZ. As SZ is a neurodevelopmental disease, the use of human-induced pluripotent stem cells (hiPSC) allows disease biology modeling while retaining the patient’s unique genetic signature. Previously, we reported a VEGFA signaling impairment in SZ-hiPSC-derived neural lineages leading to decreased angiogenesis. Here, we present a functional characterization of SZ-derived brain microvascular endothelial-like cells (BEC), the counterpart of the neurovascular crosstalk, revealing an intrinsically defective blood–brain barrier (BBB) phenotype. Transcriptomic assessment of genes related to endothelial function among three control (Ctrl BEC) and five schizophrenia patients derived BEC (SZP BEC), revealed that SZP BEC have a distinctive expression pattern of angiogenic and BBB-associated genes. Functionally, SZP BEC showed a decreased angiogenic response in vitro and higher transpermeability than Ctrl BEC. Immunofluorescence staining revealed less expression and altered distribution of tight junction proteins in SZP BEC. Moreover, SZP BEC’s conditioned media reduced barrier capacities in the brain microvascular endothelial cell line HCMEC/D3 and in an in vivo permeability assay in mice. Overall, our results describe an intrinsic failure of SZP BEC for proper barrier function. These findings are consistent with the hypothesis tracing schizophrenia origins to brain development and BBB dysfunction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Skene NG, Bryois J, Bakken TE, Breen G, Crowley JJ, Gaspar HA, et al. Genetic identification of brain cell types underlying schizophrenia. Nat Genet. 2018;50:825–33.
Potkin SG, Kane JM, Correll CU, Lindenmayer J-P, Agid O, Marder SR, et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. Npj Schizophr. 2020;6:1.
Avramopoulos D. Recent advances in the genetics of schizophrenia. Mol Neuropsychiatry. 2018;4:35–51.
Ripke S, Neale BM, Corvin A, Walters JTR, Farh KH, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7.
Stilo S, Forti M, Murray R. Environmental risk factors for schizophrenia: implications for prevention. Neuropsychiatry. 2011;1:457–66.
Brown AS. The environment and susceptibility to schizophrenia. Prog Neurobiol. 2011;93:23–58.
Birnbaum R, Weinberger DR. Genetic insights into the neurodevelopmental origins of schizophrenia. Nat Rev Neurosci. 2017;18:727–40.
Costain G, Bassett AS. Clinical applications of schizophrenia genetics: genetic diagnosis, risk, and counseling in the molecular era. Appl Clin Genet. 2012;5:1–18.
Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–93.
Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:e623–e623.
Richetto J, Meyer U. Epigenetic modifications in schizophrenia and related disorders: molecular scars of environmental exposures and source of phenotypic variability. Biol Psychiatry. 2021;89:215–26.
Najjar S, Pahlajani S, De Sanctis V, Stern JNH, Najjar A, Chong D. Neurovascular unit dysfunction and blood–brain barrier hyperpermeability contribute to schizophrenia neurobiology: a theoretical integration of clinical and experimental evidence. Front Psychiatry. 2017;8:83.
Katsel P, Roussos P, Pletnikov M, Haroutunian V. Microvascular anomaly conditions in psychiatric disease. Schizophrenia – angiogenesis connection. Neurosci Biobehav Rev. 2017;77:327–39.
Bleuler E. Dementia praecox or the group of schizophrenias. Oxford, England: International Universities Press; 1950.
Saili KS, Zurlinden TJ, Schwab AJ, Silvin A, Baker NC, Hunter ES 3rd, et al. Blood-brain barrier development: Systems modeling and predictive toxicology. Birth Defects Res. 2017;109:1680–710.
Schmidt-Kastner R, van Os J, Esquivel G, Steinbusch HWM, Rutten BPF. An environmental analysis of genes associated with schizophrenia: hypoxia and vascular factors as interacting elements in the neurodevelopmental model. Mol Psychiatry. 2012;17:1194–205.
Pong S, Karmacharya R, Sofman M, Bishop JR, Lizano P. The role of brain microvascular endothelial cell and blood-brain barrier dysfunction in schizophrenia. Complex Psychiatry. 2020;6:30–46.
Greene C, Hanley N, Campbell M. Blood-brain barrier associated tight junction disruption is a hallmark feature of major psychiatric disorders. Transl Psychiatry. 2020;10:373.
Marín-Padilla M. The human brain intracerebral microvascular system: development and structure. Front Neuroanat. 2012;6:1–14.
Ben-Zvi A, Liebner S. Developmental regulation of barrier- and non-barrier blood vessels in the CNS. J Intern Med. 2021;1:1–16.
Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. 2015;7:a020412.
Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96:17–42.
Brennand K, Savas JN, Kim Y, Tran N, Simone A, Hashimoto-Torii K, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361–8.
Hoffman GE, Schrode N, Flaherty E, Brennand KJ. New considerations for hiPSC-based models of neuropsychiatric disorders. Mol Psychiatry. 2019;24:49–66.
Ardhanareeswaran K, Mariani J, Coppola G, Abyzov A, Vaccarino FM. Human induced pluripotent stem cells for modelling neurodevelopmental disorders. Nat Rev Neurol. 2017;13:265–78.
Das D, Feuer K, Wahbeh M, Avramopoulos D. Modeling psychiatric disorder biology with stem cells. Curr Psychiatry Rep. 2020;22:24.
Moslem M, Olive J, Falk A. Stem cell models of schizophrenia, what have we learned and what is the potential? Schizophr Res. 2019;210:3–12.
Balan S, Toyoshima M, Yoshikawa T. Contribution of induced pluripotent stem cell technologies to the understanding of cellular phenotypes in schizophrenia. Neurobiol Dis. 2019;131:104162.
Casas BS, Vitória G, do Costa MN, Madeiro da Costa R, Trindade P, Maciel R, et al. hiPSC-derived neural stem cells from patients with schizophrenia induce an impaired angiogenesis. Transl Psychiatry. 2018;8:48.
Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–5.
Sochacki J, Devalle S, Reis M, de Moraes Maciel R, da Silveira Paulsen B, Brentani H, et al. Generation of iPS cell lines from schizophrenia patients using a non-integrative method. Stem Cell Res. 2016;17:97–101.
Qian T, Maguire SE, Canfield SG, Bao X, Olson WR, Shusta EV, et al. Directed differentiation of human pluripotent stem cells to blood-brain barrier endothelial cells. Sci Adv. 2017;3:48–50.
Stebbins MJ, Wilson HK, Canfield SG, Qian T, Palecek SP, Shusta EV. Differentiation and characterization of human pluripotent stem cell-derived brain microvascular endothelial cells. Methods. 2016;101:93–102.
Prieto C, Casas B, Falcón P, Villanueva A, Lois P, Lattus J, et al. Downregulation of the Netrin-1 receptor UNC5b underlies increased placental angiogenesis in human gestational diabetes mellitus. Int J Mol Sci. 2019;20:1408.
Carpentier G, Martinelli M, Courty J, Cascone I. Angiogenesis analyzer for ImageJ. In: 4th ImageJ User and Developer Conference proceedings. Mondorf-les-Bains, Luxembourg; 2012. p. 198–201. http://image.bio.methods.free.fr/ImageJ/?Angiogenesis-Analyzer-for-ImageJ&lang=en&artpage=6-6#outil_sommaire_6.
Takahashi H, Hattori S, Iwamatsu A, Takizawa H, Shibuya M. A Novel Snake Venom Vascular Endothelial Growth Factor (VEGF) predominantly induces vascular permeability through preferential signaling via VEGF Receptor-1. J Biol Chem. 2004;279:46304–14.
Brash JT, Ruhrberg C, Fantin A. Evaluating vascular hyperpermeability-inducing agents in the skin with the miles assay. J Vis Exp. 2018;136:e57524.
Schmidt-kastner R, Os JVan, Steinbusch HWM. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253–71.
Fulzele S, Pillai A. Decreased VEGF mRNA expression in the dorsolateral prefrontal cortex of schizophrenia subjects. Schizophr Res. 2009;115:372–3.
Lee B-H, Hong J-P, Hwang J-A, Ham B-J, Na K-S, Kim W-J, et al. Alterations in plasma vascular endothelial growth factor levels in patients with schizophrenia before and after treatment. Psychiatry Res. 2015;228:95–99.
Xiao W, Zhan Q, Ye F, Tang X, Li J, Dong H, et al. Baseline serum vascular endothelial growth factor levels predict treatment response to antipsychotic medication in patients with schizophrenia. Eur Neuropsychopharmacol. 2018;28:603–9.
Ye F, Zhan Q, Xiao W, Tang X, Li J, Dong H, et al. Altered serum levels of vascular endothelial growth factor in first-episode drug-naïve and chronic medicated schizophrenia. Psychiatry Res. 2018;264:361–5.
Mehlen P, Delloye-Bourgeois C, Chédotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–97.
Podjaski C, Alvarez JI, Bourbonniere L, Larouche S, Terouz S, Bin JM, et al. Netrin 1 regulates blood–brain barrier function and neuroinflammation. Brain. 2015;138:1598–612.
Prieto CP, Ortiz MC, Villanueva A, Villarroel C, Edwards SS, Elliott M, et al. Netrin-1 acts as a non-canonical angiogenic factor produced by human Wharton’s jelly mesenchymal stem cells (WJ-MSC). Stem Cell Res Ther. 2017;8:43.
Vafadari B, Salamian A, Kaczmarek L. MMP-9 in translation: from molecule to brain physiology, pathology, and therapy. J Neurochem. 2016;139:91–114.
Brilha S, Ong CWM, Weksler B, Romero N, Couraud P-O, Friedland JS. Matrix metalloproteinase-9 activity and a downregulated Hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci Rep. 2017;7:16031.
Kadry H, Noorani B, Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020;17:69.
Huang G, Osorio D, Guan J, Ji G, Cai JJ. Overdispersed gene expression in schizophrenia. Npj Schizophr. 2020;6:9.
Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–33.
Schmidt-Kastner R, Guloksuz S, Kietzmann T, van Os J, Rutten BPF. Analysis of GWAS-derived schizophrenia genes for links to ischemia-hypoxia response of the brain. Front Psychiatry. 2020;11:1–9.
Tornavaca O, Chia M, Dufton N, Almagro LO, Conway DE, Randi AM, et al. ZO-1 controls endothelial adherens junctions, cell–cell tension, angiogenesis, and barrier formation. J Cell Biol. 2015;208:821–38.
Dev KK, Henley JM. The schizophrenic faces of PICK1. Trends Pharmacol Sci. 2006;27:574–9.
Li C, Tao R, Qin W, Zheng Y, He G, Shi Y, et al. Positive association between PDLIM5 and schizophrenia in the Chinese Han population. Int J Neuropsychopharmacol. 2008;11:27–34.
Leduc-Galindo D, Qvist P, Tóth AE, Fryland T, Nielsen MS, Børglum AD, et al. The effect of hypoxia on ZEB1 expression in a mimetic system of the blood-brain barrier. Microvasc Res. 2019;122:131–5.
Li Y, Xia Y, Zhu H, Luu E, Huang G, Sun Y, et al. Investigation of neurodevelopmental deficits of 22 q11.2 deletion syndrome with a patient-iPSC-derived blood–brain barrier model. Cells. 2021;10:2576.
Pong S, Lizano P, Karmacharya R. Investigating blood-brain barrier dysfunction in schizophrenia using brain microvascular endothelial cells derived from patient-specific stem cells. Biol Psychiatry. 2020;87:S189–S190.
Crockett AM, Ryan SK, Vásquez AH, Canning C, Kanyuch N, Kebir H, et al. Disruption of the blood–brain barrier in 22q11.2 deletion syndrome. Brain. 2021;144:1351–60.
Hashimoto K, Shimizu E, Komatsu N, Nakazato M, Okamura N, Watanabe H, et al. Increased levels of serum basic fibroblast growth factor in schizophrenia. Psychiatry Res. 2003;120:211–8.
Greene C, Kealy J, Humphries MM, Gong Y, Hou J, Hudson N, et al. Dose-dependent expression of claudin-5 is a modifying factor in schizophrenia. Mol Psychiatry. 2018;23:2156–66.
Kis B, Chen L, Ueta Y, Busija DW. Autocrine peptide mediators of cerebral endothelial cells and their role in the regulation of blood–brain barrier. Peptides. 2006;27:211–22.
Vermeer PD, Denker J, Estin M, Moninger TO, Keshavjee S, Karp P, et al. MMP9 modulates tight junction integrity and cell viability in human airway epithelia. Am J Physiol Cell Mol Physiol. 2009;296:L751–L762.
Domenici E, Willé DR, Tozzi F, Prokopenko I, Miller S, McKeown A, et al. Plasma protein biomarkers for depression and schizophrenia by multi analyte profiling of case-control collections. PLoS ONE. 2010;5:e9166.
Yamamori H, Hashimoto R, Ishima T, Kishi F, Yasuda Y, Ohi K, et al. Plasma levels of mature brain-derived neurotrophic factor (BDNF) and matrix metalloproteinase-9 (MMP-9) in treatment-resistant schizophrenia treated with clozapine. Neurosci Lett. 2013;556:37–41.
Chang S-H, Chiang S-Y, Chiu C-C, Tsai C-C, Tsai H-H, Huang C-Y, et al. Expression of anti-cardiolipin antibodies and inflammatory associated factors in patients with schizophrenia. Psychiatry Res. 2011;187:341–6.
Han H, He X, Tang J, Liu W, Liu K, Zhang J, et al. The C(−1562)T polymorphism of matrix metalloproteinase-9 gene is associated with schizophrenia in China. Psychiatry Res. 2011;190:163–4.
Rybakowski JK, Skibinska M, Kapelski P, Kaczmarek L, Hauser J. Functional polymorphism of the matrix metalloproteinase-9 (MMP-9) gene in schizophrenia. Schizophr Res. 2009;109:90–93.
Wiera G, Wozniak G, Bajor M, Kaczmarek L, Mozrzymas JW. Maintenance of long-term potentiation in hippocampal mossy fiber-CA3 pathway requires fine-tuned MMP-9 proteolytic activity. Hippocampus. 2013;23:529–43.
Glasgow SD, Ruthazer ES, Kennedy TE. Guiding synaptic plasticity: novel roles for netrin-1 in synaptic plasticity and memory formation in the adult brain. J Physiol. 2021;599:493–505.
Glasgow SD, Labrecque S, Beamish IV, Aufmkolk S, Gibon J, Han D, et al. Activity-dependent Netrin-1 secretion drives synaptic insertion of GluA1-containing AMPA receptors in the Hippocampus. Cell Rep. 2018;25:168–.e6.
Bayas A, Hummel V, Kallmann BA, Karch C, Toyka KV, Rieckmann P. Human cerebral endothelial cells are a potential source for bioactive BDNF. Cytokine. 2002;19:55–58.
Nakahashi T, Fujimura H, Altar CA, Li J, Kambayashi JI, Tandon NN, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–7.
Yi H, Hu J, Qian J, Hackam AS. Expression of brain-derived neurotrophic factor is regulated by the Wnt signaling pathway. Neuroreport. 2012;23:189–94.
Wu H, Lu D, Jiang H, Xiong Y, Qu C, Li B, et al. Simvastatin-mediated upregulation of VEGF and BDNF, activation of the PI3K/Akt pathway, and increase of neurogenesis are associated with therapeutic improvement after traumatic brain injury. J Neurotrauma. 2008;25:130–9.
Lu TM, Houghton S, Magdeldin T, Durán JGB, Minotti AP, Snead A, et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc Natl Acad Sci USA 2021;118:e2016950118.
Lippmann ES, Azarin SM, Palecek SP, Shusta EV. Commentary on human pluripotent stem cell-based blood–brain barrier models. Fluids Barriers CNS. 2020;17:64.
Acknowledgements
We thank Dr. Carol San Martin for kindly donating the HCMEC/D3 line. We are grateful to all the Steven Rehen’s laboratory crew, especially to Dr. Livia Goto, Ismael Gomez, and Scarlett Mercante for technical support. We thank Sofía Puvogel and Robert Seitter for critical reading of the manuscript and Dr. Emiliano Molina for technical assistance. Intramural grants provided from the D’Or Institute for Research and Education (IDOR). Funding from ANID Fondecyt # 1190083 and Fondecyt # 1221522 (VP) & Conicyt # 21150781 (BSC).
Author information
Authors and Affiliations
Contributions
BSC and VP conceived and designed the experiments. BSC, GV, CPP, MC, CC, and MU performed the experiments and/or contributed to data acquisition. BSC and VP analyzed and interpreted the data. BSC wrote the original article. CPP, MC, SKR, FE, ME, and VP critically revised and edited the article. VP, FE, ME, and SR provided resources, and VP was responsable for general supervision. All authors approved the final version of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Casas, B.S., Vitória, G., Prieto, C.P. et al. Schizophrenia-derived hiPSC brain microvascular endothelial-like cells show impairments in angiogenesis and blood–brain barrier function. Mol Psychiatry 27, 3708–3718 (2022). https://doi.org/10.1038/s41380-022-01653-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01653-0
This article is cited by
-
Schizophrenia endothelial cells exhibit higher permeability and altered angiogenesis patterns in patient-derived organoids
Translational Psychiatry (2024)
-
A network of transcriptomic signatures identifies novel comorbidity mechanisms between schizophrenia and somatic disorders
Discover Mental Health (2024)
-
Formulating treatment of major psychiatric disorders: algorithm targets the dominantly affected brain cell-types
Discover Mental Health (2023)