Abstract
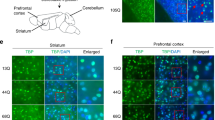
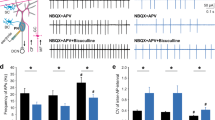
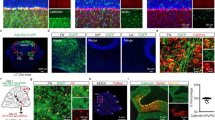
Cerebellar deficits with Purkinje cell (PCs) loss are observed in several neurologic disorders. However, the underlying mechanisms as to how the cerebellum is affected during development remain unclear. Here we demonstrated that specific inactivation of murine Ebp1 in the central nervous system causes a profound neuropathology characterized by reduced cerebellar volume and PCs loss with abnormal dendritic development, leading to phenotypes including motor defects and schizophrenia (SZ)-like behaviors. Loss of Ebp1 leads to untimely gene expression of Fbxw7, an E3 ubiquitin ligase, resulting in aberrant protein degradation of PTF1A, thereby eliciting cerebellar defects. Reinstatement of Ebp1, but not the Ebp1-E183Ter mutant found in SZ patients, reconstituted cerebellar architecture with increased PCs numbers and improved behavioral phenotypes. Thus, our findings indicate a crucial role for EBP1 in cerebellar development, and define a molecular basis for the cerebellar contribution to neurologic disorders such as SZ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Liu Z, Ahn J-Y, Liu X, Ye K. Ebp1 isoforms distinctively regulate cell survival and differentiation. Proc Natl Acad Sci. 2006;103:10917–22.
Ahn JY, Liu X, Cheng D, Peng J, Chan PK, Wade PA, et al. Nucleophosmin/B23, a nuclear PI(3,4,5)P(3) receptor, mediates the antiapoptotic actions of NGF by inhibiting CAD. Mol Cell. 2005;18:435–45.
Ko HR, Kim CK, Lee SB, Song J, Lee KH, Kim KK, et al. P42 Ebp1 regulates the proteasomal degradation of the p85 regulatory subunit of PI3K by recruiting a chaperone-E3 ligase complex HSP70/CHIP. Cell Death Dis. 2014;5:e1131–e.
Hwang I, Ko HR, Ahn J-Y. The roles of multifunctional protein ErbB3 binding protein 1 (EBP1) isoforms from development to disease. Exp Mol Med. 2020;52:1039–47.
Ko HR, Hwang I, Ahn SY, Chang YS, Park WS, Ahn J-Y. Neuron-specific expression of p48 Ebp1 during murine brain development and its contribution to CNS axon regeneration. BMB Rep. 2017;50:126–31.
Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13:619–35.
Ko HR, Hwang I, Jin E-J, Yun T, Ryu D, Kang J-S, et al. Roles of ErbB3-binding protein 1 (EBP1) in embryonic development and gene-silencing control. Proc Natl Acad Sci. 2019;116:24852–60.
Kim BS, Ko HR, Hwang I, Cho SW, Ahn JY. EBP1 regulates Suv39H1 stability via the ubiquitin-proteasome system in neural development. BMB Rep. 2021;54:413–8.
Seto Y, Nakatani T, Masuyama N, Taya S, Kumai M, Minaki Y, et al. Temporal identity transition from Purkinje cell progenitors to GABAergic interneuron progenitors in the cerebellum. Nat Commun. 2014;5:3337.
Pascual M, Abasolo I, Mingorance-Le Meur A, Martínez A, Del Rio JA, Wright CV, et al. Cerebellar GABAergic progenitors adopt an external granule cell-like phenotype in the absence of Ptf1a transcription factor expression. Proc Natl Acad Sci USA. 2007;104:5193–8.
Millen KJ, Steshina EY, Iskusnykh IY, Chizhikov VV. Transformation of the cerebellum into more ventral brainstem fates causes cerebellar agenesis in the absence of Ptf1a function. Proc Natl Acad Sci. 2014;111:E1777–E1786.
Sellick GS, Barker KT, Stolte-Dijkstra I, Fleischmann C, Coleman RJ, Garrett C, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–5.
Zhao Y, Kwan KM, Mailloux CM, Lee WK, Grinberg A, Wurst W, et al. LIM-homeodomain proteins Lhx1 and Lhx5, and their cofactor Ldb1, control Purkinje cell differentiation in the developing cerebellum. Proc Natl Acad Sci USA. 2007;104:13182–6.
Lui NC, Tam WY, Gao C, Huang J-D, Wang CC, Jiang L, et al. Lhx1/5 control dendritogenesis and spine morphogenesis of Purkinje cells via regulation of Espin. Nat Commun. 2017;8:15079.
Carlson KM, Andresen JM, Orr HT. Emerging pathogenic pathways in the spinocerebellar ataxias. Curr Opin Genet Dev. 2009;19:247–53.
Picard H, Amado I, Mouchet-Mages S, Olié J-P, Krebs M-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophrenia Bull. 2008;34:155–72.
Mavroudis IA, Petrides F, Manani M, Chatzinikolaou F, Ciobică AS, Pădurariu M, et al. Purkinje cells pathology in schizophrenia. A morphometric approach. Rom J Morphol Embryol. 2017;58:419–24.
Moberget T, Doan NT, Alnæs D, Kaufmann T, Córdova-Palomera A, Lagerberg TV, et al. Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry. 2018;23:1512–20.
Koepp DM, Schaefer LK, Ye X, Keyomarsi K, Chu C, Harper JW, et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science. 2001;294:173–7.
Minella AC, Clurman BE. Mechanisms of tumor suppression by the SCF(Fbw7). Cell Cycle. 2005;4:1356–9.
Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374–8.
Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP). Genes Dev. 2010;24:72–85.
Jandke A, Da Costa C, Sancho R, Nye E, Spencer-Dene B, Behrens A. The F-box protein Fbw7 is required for cerebellar development. Dev Biol. 2011;358:201–12.
Kipreos ET, Pagano M. The F-box protein family. Genome Biol. 2000;1:Reviews3002.
Betz UA, Vosshenrich CA, Rajewsky K, Müller W. Bypass of lethality with mosaic mice generated by Cre-loxP-mediated recombination. Curr Biol. 1996;6:1307–16.
Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103.
Wildner H, Das Gupta R, Bröhl D, Heppenstall PA, Zeilhofer HU, Birchmeier C. Genome-Wide Expression Analysis of Ptf1a- and Ascl1-Deficient Mice Reveals New Markers for Distinct Dorsal Horn Interneuron Populations Contributing to Nociceptive Reflex Plasticity. J. Neurosci. 2013;33:7299–307.
Chang JC, Meredith DM, Mayer PR, Borromeo MD, Lai HC, Ou Y-H et al. Prdm13 mediates the balance of inhibitory and excitatory neurons in somatosensory circuits. Dev Cell. 2013;25:182–95.
Kim YS, Lee SY, Yoon JW, Kim D, Yu S, Kim JS, et al. Cardiotoxicity induced by the combination therapy of chloroquine and azithromycin in human embryonic stem cell-derived cardiomyocytes. BMB Rep. 2020;53:545–50.
Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer. 2008;8:83–93.
Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, Skrzypek E. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 2015;43:D512–20.
Park H-M, Kim H, Lee K-H, Cho J-Y. Analysis of opposing histone modifications H3K4me3 and H3K27me3 reveals candidate diagnostic biomarkers for TNBC and gene set prediction combination. BMB Rep. 2020;53:266–71.
Kim J, Jung E, Ahn SS, Yeo H, Lee JY, Seo JK, et al. WNT11 is a direct target of early growth response protein 1. BMB Rep. 2020;53:628–33.
Finnin MS, Donigian JR, Cohen A, Richon VM, Rifkind RA, Marks PA, et al. Structures of a histone deacetylase homologue bound to the TSA and SAHA inhibitors. Nature. 1999;401:188–93.
Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52.
Wang Y, Zhang P, Wang Y, Zhan P, Liu C, Mao JH, et al. Distinct interactions of EBP1 Isoforms with FBXW7 elicits different functions in cancer. Cancer Res. 2017;77:1983–96.
Lungu O, Barakat M, Laventure S, Debas K, Proulx S, Luck D, et al. The incidence and nature of cerebellar findings in schizophrenia: a quantitative review of fMRI literature. Schizophr Bull. 2013;39:797–806.
Moberget T, Ivry RB. Prediction, psychosis, and the cerebellum. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2019;4:820–31.
Kühn S, Romanowski A, Schubert F, Gallinat J. Reduction of cerebellar grey matter in Crus I and II in schizophrenia. Brain Struct Funct. 2012;217:523–9.
Walther S, Strik W. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66:77–92.
Quinn M, McHugo M, Armstrong K, Woodward N, Blackford J, Heckers S. Impact of substance use disorder on gray matter volume in schizophrenia. Psychiatry Res Neuroimaging. 2018;280:9–14.
Wolfers T, Doan NT, Kaufmann T, Alnæs D, Moberget T, Agartz I, et al. Mapping the heterogeneous phenotype of schizophrenia and bipolar disorder using normative models. JAMA Psychiatry. 2018;75:1146–55.
Ding Y, Ou Y, Pan P, Shan X, Chen J, Liu F, et al. Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging. 2019;283:24–33.
Peralta V, Cuesta MJ. Motor features in psychotic disorders. I. Factor structure and clinical correlates. Schizophr Res. 2001;47:107–16.
Peralta V, de Jalón EG, Campos MS, Basterra V, Sanchez-Torres A, Cuesta MJ. Risk factors, pre-morbid functioning and episode correlates of neurological soft signs in drug-naive patients with schizophrenia-spectrum disorders. Psychological Med. 2011;41:1279–89.
Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169:217–33.
Tsai PT, Hull C, Chu Y, Greene-Colozzi E, Sadowski AR, Leech JM, et al. Autistic-like behaviour and cerebellar dysfunction in Purkinje cell Tsc1 mutant mice. Nature. 2012;488:647–51.
Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 2014;505:361–6.
Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48:10–18.
Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Jama. 2002;288:1740–8.
Acknowledgements
We are thankful to the Institute for Basic Science (IBS), Center for Neuroscience Imaging Research (IBS-R015-D1-2016-a00) for providing access to animal scanner and professional, technical support. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government [Ministry of Science, Information and Communication Technology and Future Planning (MSIP)] (2016R1A5A2945889 and 2020R1A2C2003268).
Author information
Authors and Affiliations
Contributions
Designed and analyzed the study, IH and J-YA; Performed Experiments, IH, B-SK, H-RK, SC, and SS; Performed MRI, IH, and H-YL; Analyzed Results, IH, S-WC, DR, and J-YA; Wrote Manuscript, J-YA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hwang, I., Kim, BS., Ko, H.R. et al. Cerebellar dysfunction and schizophrenia-like behavior in Ebp1-deficient mice. Mol Psychiatry 27, 2030–2041 (2022). https://doi.org/10.1038/s41380-022-01458-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-022-01458-1