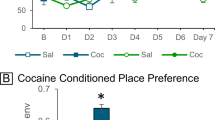
Abstract
Cocaine exerts its stimulant effect by inhibiting dopamine reuptake leading to increased dopamine signaling. This action is thought to reflect binding of cocaine to the dopamine transporter (DAT) to inhibit its function. However, cocaine is a relatively weak inhibitor of DAT, and many DAT inhibitors do not share the behavioral actions of cocaine. We previously showed that toxic levels of cocaine induce autophagic neuronal cell death. Here, we show that subnanomolar concentrations of cocaine elicit neural autophagy in vitro and in vivo. Autophagy inhibitors reduce the locomotor stimulant effect of cocaine in mice. Cocaine-induced autophagy degrades transporters for dopamine but not serotonin in the nucleus accumbens. Autophagy inhibition impairs cocaine conditioned place preference in mice. Our findings indicate that autophagic degradation of DAT modulates behavioral actions of cocaine.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its Supplementary Data files).
References
Hall FS, Sora I, Drgonova J, Li X-F, Goeb M, Uhl GR. Molecular mechanisms underlying the rewarding effects of cocaine. Ann N Y Acad Sci. 2004;1025:47–56.
Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharm. 2003;479:23–40.
Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85.
Edwards DJ, Bowles SK. Protein binding of cocaine in human serum. Pharm Res. 1988;5:440–2.
Guha P, Harraz MM, Snyder SH. Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proc Natl Acad Sci USA. 2016;113:1417–22.
Majewska MD. Neurotoxicity and neuropathology associated with chronic cocaine abuse. NIDA Res Monogr. 1996;162:70–72.
CLARK SL. Cellular differentiation in the kidneys of newborn mice studies with the electron microscope. J Biophys Biochem Cytol. 1957;3:349–62.
Deter RL, de Duve C. Influence of glucagon, an inducer of cellular autophagy, on some physical properties of rat liver lysosomes. J Cell Biol. 1967;33:437–49.
Dent P, Booth L, Poklepovic A, Hancock JF. Signaling alterations caused by drugs and autophagy. Cell Signal. 2019;64:109416.
Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12:1–222.
Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15:1101–11.
Rayport S, Sulzer D, Shi WX, Sawasdikosol S, Monaco J, Batson D, et al. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J Neurosci. 1992;12:4264–80.
Harraz MM, Eacker SM, Wang X, Dawson TM, Dawson VL. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc Natl Acad Sci USA. 2012;109:18962–7.
Graham JM. Isolation of lysosomes from tissues and cells by differential and density gradient centrifugation. Curr Protoc Cell Biol. 2001;Chapter 3:Unit 3.6.
Tanda G, Newman AH, Ebbs AL, Tronci V, Green JL, Tallarida RJ, et al. Combinations of cocaine with other dopamine uptake inhibitors: assessment of additivity. J Pharm Exp Ther. 2009;330:802–9.
Mereu M, Tronci V, Chun LE, Thomas AM, Green JL, Katz JL, et al. Cocaine-induced endocannabinoid release modulates behavioral and neurochemical sensitization in mice. Addict Biol. 2015;20:91–103.
Keighron JD, Quarterman JC, Cao J, DeMarco EM, Coggiano MA, Gleaves A, et al. Effects of (R)-modafinil and modafinil analogues on dopamine dynamics assessed by voltammetry and microdialysis in the mouse nucleus accumbens shell. ACS Chem Neurosci. 2019;10:2012–21.
Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Compact third eddition. San Diego, CA: Academic Press; 2008.
Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–64.
Janowsky A, Neve K, Eshleman AJ. Uptake and release of neurotransmitters. Curr Protoc Neurosci 2001;Chapter 7:Unit7.9–7.9.22.
Egan DF, Chun MGH, Vamos M, Zou H, Rong J, Miller CJ, et al. Small molecule inhibition of the autophagy kinase ULK1 and identification of ULK1 substrates. Mol Cell. 2015;59:285–97.
Lu Y, Dong S, Hao B, Li C, Zhu K, Guo W, et al. Vacuolin-1 potently and reversibly inhibits autophagosome-lysosome fusion by activating RAB5A. Autophagy. 2014;10:1895–905.
Degtyarev M, De Mazière A, Orr C, Lin J, Lee BB, Tien JY, et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J Cell Biol. 2008;183:101–16.
Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–26.
Maday S, Wallace KE, Holzbaur ELF. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol. 2012;196:407–17.
Maday S, Holzbaur ELF. Autophagosome biogenesis in primary neurons follows an ordered and spatially regulated pathway. Dev Cell. 2014;30:71–85.
Hill SE, Kauffman KJ, Krout M, Richmond JE, Melia TJ, Colón-Ramos DA. Maturation and clearance of autophagosomes in neurons depends on a specific cysteine protease isoform, ATG-4.2. Dev Cell. 2019;49:251–266.e8.
WHITBY LG, HERTTING G, AXELROD J. Effect of cocaine on the disposition of noradrenaline labelled with tritium. Nature. 1960;187:604–5.
Kilty JE, Lorang D, Amara SG. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254:578–9.
Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, et al. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–8.
Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96.
Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–23.
Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, et al. Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci USA. 1993;90:2542–6.
Blakely RD, Bauman AL. Biogenic amine transporters: regulation in flux. Curr Opin Neurobiol. 2000;10:328–36.
Cole JO, Levin A, Beake B, Kaiser PE, Scheinbaum ML. Sibutramine: a new weight loss agent without evidence of the abuse potential associated with amphetamines. J Clin Psychopharmacol. 1998;18:231–6.
Schuh LM, Schuster CR, Hopper JA, Mendel CM. Abuse liability assessment of sibutramine, a novel weight control agent. Psychopharmacology. 2000;147:339–46.
Peck AW, Bye CE, Clubley M, Henson T, Riddington C. A comparison of bupropion hydrochloride with dexamphetamine and amitriptyline in healthy subjects. Br J Clin Pharm. 1979;7:469–78.
Chait LD, Uhlenhuth EH, Johanson CE. Reinforcing and subjective effects of several anorectics in normal human volunteers. J Pharm Exp Ther. 1987;242:777–83.
Chait LD, Uhlenhuth EH, Johanson CE. The discriminative stimulus and subjective effects of phenylpropanolamine, mazindol and d-amphetamine in humans. Pharm Biochem Behav. 1986;24:1665–72.
Schoedel KA, Meier D, Chakraborty B, Manniche PM, Sellers EM. Subjective and objective effects of the novel triple reuptake inhibitor tesofensine in recreational stimulant users. Clin Pharm Ther. 2010;88:69–78.
Post RM, Kotin J, Goodwin FK. The effects of cocaine on depressed patients. Am J Psychiatry. 1974;131:511–7.
Uhl GR. Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov Disord. 2003;18:S71–80.
Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–12.
Sora I, Wichems C, Takahashi N, Li XF, Zeng Z, Revay R, et al. Cocaine reward models: conditioned place preference can be established in dopamine- and in serotonin-transporter knockout mice. Proc Natl Acad Sci USA. 1998;95:7699–704.
Sora I, Hall FS, Andrews AM, Itokawa M, Li XF, Wei HB, et al. Molecular mechanisms of cocaine reward: combined dopamine and serotonin transporter knockouts eliminate cocaine place preference. Proc Natl Acad Sci USA. 2001;98:5300–5.
Rao A, Sorkin A, Zahniser NR. Mice expressing markedly reduced striatal dopamine transporters exhibit increased locomotor activity, dopamine uptake turnover rate, and cocaine responsiveness. Synapse. 2013;67:668–77.
Tilley MR, Cagniard B, Zhuang X, Han DD, Tiao N, Gu HH. Cocaine reward and locomotion stimulation in mice with reduced dopamine transporter expression. BMC Neurosci. 2007;8:42–7.
Chen R, Tilley MR, Wei H, Zhou F, Zhou F-M, Ching S, et al. Abolished cocaine reward in mice with a cocaine-insensitive dopamine transporter. Proc Natl Acad Sci USA. 2006;103:9333–8.
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–9.
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J-I, Tanida I, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–4.
Valente MJ, Amaral C, Correia-da-Silva G, Duarte JA, Bastos M, de L, et al. Methylone and MDPV activate autophagy in human dopaminergic SH-SY5Y cells: a new insight into the context of β-keto amphetamines-related neurotoxicity. Arch Toxicol. 2017;91:3663–76.
Mercer LD, Higgins GC, Lau CL, Lawrence AJ, Beart PM. MDMA-induced neurotoxicity of serotonin neurons involves autophagy and rilmenidine is protective against its pathobiology. Neurochem Int. 2017;105:80–90.
Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–60.
Acknowledgements
We thank L. Hester, R. Barrow, A. Snowman, S. McTeer, and L. Albacarys from the SHS laboratory for their assistance. We thank B. Smith and the Microscope Core Facility at the Institute for Basic Biomedical Sciences, Johns Hopkins School of Medicine, for helping in preparation of the TEM samples. We are also grateful for fruitful discussions with members of the SHS Laboratory.
Funding
This work was supported by U.S. Public Health Service Grants DA00266 and DA044123 to SHS and a NARSAD Young Investigator Grant (# 25360) from the Brain & Behavior Foundation to MMH. Support for this research was provided in part by the National Institute on Drug Abuse—Intramural Research Program, NIH/DHHS (Z1A DA000611).
Author information
Authors and Affiliations
Contributions
MMH, PG, PC, IGK, ERS, YJS, LR, IT, APM, MAC, and VV performed experiments. MMH, PG, GT, RR, and SHS designed experiments, analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Harraz, M.M., Guha, P., Kang, I.G. et al. Cocaine-induced locomotor stimulation involves autophagic degradation of the dopamine transporter. Mol Psychiatry 26, 370–382 (2021). https://doi.org/10.1038/s41380-020-00978-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00978-y