Abstract
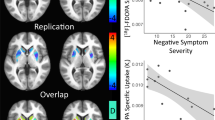
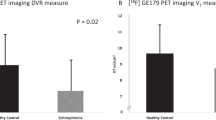
Patients at clinical high-risk (CHR) for psychosis show elevations in [18F]DOPA uptake, an estimate of dopamine (DA) synthesis capacity, in the striatum predictive of conversion to schizophrenia. Intrasynaptic DA levels can be inferred from imaging the change in radiotracer binding at D2 receptors due to a pharmacological challenge. Here, we used methylphenidate, a DA reuptake inhibitor, and [11C]-(+)-PHNO, to measure synaptic DA availability in CHR both in striatal and extra-striatal brain regions. Fourteen unmedicated, nonsubstance using CHR individuals and 14 matched control subjects participated in the study. Subjects underwent two [11C]-(+)-PHNO scans, one at baseline and one following administration of a single oral dose (60 mg) of methylphenidate. [11C]-(+)-PHNO BPND, the binding potential relative to the nondisplaceable compartment, was derived using the simplified reference tissue model with cerebellum as reference tissue. The percent change in BPND between scans, ΔBPND, was computed as an index of synaptic DA availability, and group comparisons were performed with a linear mixed model. An overall trend was found for greater synaptic DA availability (∆BPND) in CHR than controls (p = 0.06). This was driven entirely by ∆BPND in ventral striatum (−34 ± 14% in CHR, −20 ± 12% in HC; p = 0.023). There were no significant group differences in any other brain region. There were no significant differences in DA transmission in any striatal region between converters and nonconverters, although this finding is limited by the small sample size (N = 2). There was a strong and negative correlation between ΔBPND in VST and severity of negative symptoms at baseline in the CHR group (r = −0.66, p < 0.01). We show abnormally increased DA availability in the VST in CHR and an inverse relationship with negative symptoms. Our results suggest a potential early role for mesolimbic dopamine overactivity in CHR. Longitudinal studies are needed to ascertain the significance of the differential topography observed here with the [18F]DOPA literature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fusar-Poli P, Borgwardt S, Bechdolf A, Addington J, Riecher-Rossler A, Schultze-Lutter F, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70:107–20.
Addington J, Cornblatt BA, Cadenhead KS, Cannon TD, McGlashan TH, Perkins DO, et al. At clinical high risk for psychosis: outcome for nonconverters. Am J Psychiatry. 2011;168:800–5.
Howes OD, Bose SK, Turkheimer F, Valli I, Egerton A, Valmaggia LR, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;168:1311–7.
Egerton A, Chaddock CA, Winton-Brown TT, Bloomfield MA, Bhattacharyya S, Allen P, et al. Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol Psychiatry. 2013;74:106–12.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, et al. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20.
Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-specific dopamine abnormalities in schizophrenia. Biol Psychiatry. 2017;81:31–42.
Howes O, Bose S, Turkheimer F, Valli I, Egerton A, Stahl D, et al. Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol Psychiatry. 2011;16:885–6.
Bloemen OJ, de Koning MB, Gleich T, Meijer J, de Haan L, Linszen DH, et al. Striatal dopamine D2/3 receptor binding following dopamine depletion in subjects at ultra high risk for psychosis. Eur Neuropsychopharmacol. 2013;23:126–32.
Allen P, Luigjes J, Howes OD, Egerton A, Hirao K, Valli I, et al. Transition to psychosis associated with prefrontal and subcortical dysfunction in ultra high-risk individuals. Schizophr Bull. 2012;38:1268–76.
Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121.
Ginovart N, Willeit M, Rusjan P, Graff A, Bloomfield PM, Houle S, et al. Positron emission tomography quantification of [11C]-(+)-PHNO binding in the human brain. J Cereb Blood Flow Metab. 2007;27:857–71.
Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–60.
Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–9.
Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, et al. In vivo binding of antipsychotics to D(3) and D(2) receptors: a PET study in baboons with [(11)C]-(+)-PHNO. Neuropsychopharmacology. 2011;36:887–95.
Boileau I, Payer D, Chugani B, Lobo DS, Houle S, Wilson AA, et al. In vivo evidence for greater amphetamine-induced dopamine release in pathological gambling: a positron emission tomography study with [(11)C]-(+)-PHNO. Mol Psychiatry. 2013;19:1305–13.
Gallezot JD, Kloczynski T, Weinzimmer D, Labaree D, Zheng MQ, Lim K, et al. Imaging nicotine- and amphetamine-induced dopamine release in rhesus monkeys with [(11)C]PHNO vs [(11)C]raclopride PET. Neuropsychopharmacology. 2014;39:866–74.
Halldin C, Farde L, Hogberg T, Mohell N, Hall H, Suhara T, et al. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med. 1995;36:1275–81.
Narendran R, Frankle WG, Mason NS, Rabiner EA, Gunn RN, Searle GE, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–61.
Chou YH, Halldin C, Farde L. Effect of amphetamine on extrastriatal D2 dopamine receptor binding in the primate brain: a PET study. Synapse. 2000;38:138–43.
Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–73.
Narendran R, Mason NS, May MA, Chen CM, Kendro S, Ridler K, et al. Positron emission tomography imaging of dopamine D2/3 receptors in the human cortex with [(1)(1)C]FLB 457: reproducibility studies. Synapse. 2011;65:35–40.
Sudo Y, Suhara T, Inoue M, Ito H, Suzuki K, Saijo T, et al. Reproducibility of [11 C]FLB 457 binding in extrastriatal regions. Nucl Med Commun. 2001;22:1215–21.
Mizrahi R, Addington J, Rusjan PM, Suridjan I, Ng A, Boileau I, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–7.
Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, et al. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39:1479–89.
Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29:703–15.
McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson LA. Scale for the assessment of prodromal symptoms and states. In: Miller T, Mednick, SA, McGlashan, TH, Liberger, J, Johannessen, JO, editor Early intervention in psychotic disorders. Dordrecht: Kluwer Academic Publishers; 2001. p. 135–49.
Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the prodrome for schizophrenia: preliminary evidence of interrater reliability and predictive validity using operational criteria and a structured interview. Am J Psychiatry. 2002;159:863–65.
Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Cannon T, Ventura J, et al. Prodromal assessment with the Sstructured Interview for Prodromal Syndromes: Predictive validity, interrater reliability, and training to reliability. Schizophrenia Bull. 2003;29:703–15.
Rosen JL, Woods SW, Miller TJ, McGlashan TH. Prospective observations of emerging psychosis. J Nerv Ment Dis. 2002;190:133–41.
McGlashan TH, Walsh BC, Woods SW. Structured Interview for Psychosis Risk Syndromes. New Haven, CT: PRIME Research Clinic, Yale School of Medicine; 2014.
Nurnberger JI Jr, Blejar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. Arch Gen Psychiatry. 1994;51:849–59.
First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research NYSPI; 2002.
Guy W. Clinical global impressions. In: Guy W, editor. ECDEU assessment manual for psychopharmacology: revised, ADM 76-338. Washington, DC: Department of Health, Education, and Welfare; 1976. p. 217–22.
Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–41.
SPM Online Bibliography. http://www.fil.ion.ucl.ac.uk/spm/doc/biblio/. Accessed 2020.
Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–9.
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC. nlme: linear and nonlinear fixed effects models. 2020. https://CRAN.R-project.org/package=nlme.
Nestler EJ, Carlezon WA Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–9.
Suridjan I, Rusjan P, Addington J, Wilson AA, Houle S, Mizrahi R. Dopamine D2 and D3 binding in people at clinical high risk for schizophrenia, antipsychotic-naive patients and healthy controls while performing a cognitive task. J Psychiatry Neurosci. 2013;38:98–106.
McCutcheon RA, Abi-Dargham A, Howes OD. Schizophrenia, dopamine and the striatum: from biology to symptoms. Trends Neurosci. 2019;42:205–20.
Martinez D, Slifstein M, Broft A, Mawlawi O, Hwang DR, Huang Y, et al. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J Cereb Blood Flow Metab. 2003;23:285–300.
Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology. 1987;91:415–33.
Simpson EH, Kellendonk C. Insights about striatal circuit function and schizophrenia from a mouse model of dopamine D2 receptor upregulation. Biol Psychiatry. 2017;81:21–30.
Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–86.
Schobel SA, Chaudhury NH, Khan UA, Paniagua B, Styner MA, Asllani I, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78:81–93.
Schobel SA, Lewandowski NM, Corcoran CM, Moore H, Brown T, Malaspina D, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66:938–46.
Ho NF, Holt DJ, Cheung M, Iglesias JE, Goh A, Wang M, et al. Progressive decline in hippocampal CA1 volume in individuals at ultra-high-risk for psychosis who do not remit: findings from the longitudinal youth at risk study. Neuropsychopharmacology. 2017;42:1361–70.
Bossong MG, Antoniades M, Azis M, Samson C, Quinn B, Bonoldi I, et al. Association of hippocampal glutamate levels with adverse outcomes in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2019;76:199–207.
Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–302.
Provenzano FA, Guo J, Wall MM, Feng X, Sigmon HC, Brucato G, et al. Hippocampal pathology in clinical high-risk patients and the onset of schizophrenia. Biol Psychiatry. 2020;87:234–42.
Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12:585–601.
Kegeles LS, Abi-Dargham A, Frankle WG, Gil R, Cooper TB, Slifstein M, et al. Increased synaptic dopamine function in associative regions of the striatum in schizophrenia. Arch Gen Psychiatry. 2010;67:231–9.
Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38.
Parsey RV, Oquendo MA, Zea-Ponce Y, Rodenhiser J, Kegeles LS, Pratap M, et al. Dopamine D(2) receptor availability and amphetamine-induced dopamine release in unipolar depression. Biol Psychiatry. 2001;50:313–22.
Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA. Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse. 2009;63:681–9.
Schneier FR, Slifstein M, Whitton AE, Pizzagalli DA, Reinen J, McGrath PJ, et al. Dopamine release in antidepressant-naive major depressive disorder: a multimodal [(11)C]-(+)-PHNO positron emission tomography and functional magnetic resonance imaging study. Biol Psychiatry. 2018;84:563–73.
Slifstein M, Narendran R, Hwang DR, Sudo Y, Talbot PS, Huang YY. et al. Effect of amphetamine on [F-18]fallypride in vivo binding to D-2 receptors in striatal and extrastriatal regions of the primate brain: Single bolus and bolus plus constant infusion studies. Synapse. 2004;54:46–63.
Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012;66:489–500.
Slifstein M, Abi-Dargham A, Girgis RR, Suckow RF, Cooper TB, Divgi CR, et al. Binding of the D3-preferring antipsychotic candidate F17464 to dopamine D3 and D2 receptors: a PET study in healthy subjects with [(11)C]-(+)-PHNO. Psychopharmacology. 2020;237:519–27.
Berry AS, Shah VD, Furman DJ, White RL 3rd, Baker SL, O’Neil JP, et al. Dopamine synthesis capacity is associated with D2/3 receptor binding but not dopamine release. Neuropsychopharmacology. 2018;43:1201–11.
Nour MM, McCutcheon R, Howes OD. The relationship between dopamine synthesis capacity and release: implications for psychosis. Neuropsychopharmacology. 2018;43:1195–96.
Kumakura Y, Cumming P, Vernaleken I, Buchholz HG, Siessmeier T, Heinz A, et al. Elevated [18F]fluorodopamine turnover in brain of patients with schizophrenia: an [18F]fluorodopa/positron emission tomography study. J Neurosci. 2007;27:8080–7.
Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–24.
Schifani C, Tseng HH, Kenk M, Tagore A, Kiang M, Wilson AA, et al. Cortical stress regulation is disrupted in schizophrenia but not in clinical high risk for psychosis. Brain. 2018;141:2213–24.
Tseng HH, Watts JJ, Kiang M, Suridjan I, Wilson AA, Houle S, et al. Nigral stress-induced dopamine release in clinical high risk and antipsychotic-naive schizophrenia. Schizophr Bull. 2018;44:542–51.
Cassidy CM, Zucca FA, Girgis RR, Baker SC, Weinstein JJ, Sharp ME, et al. Neuromelanin-sensitive MRI as a noninvasive proxy measure of dopamine function in the human brain. Proc Natl Acad Sci USA. 2019;116:5108–17.
Brucato G, Masucci MD, Arndt LY, Ben-David S, Colibazzi T, Corcoran CM, et al. Baseline demographics, clinical features and predictors of conversion among 200 individuals in a longitudinal prospective psychosis-risk cohort. Psychol Med. 2017;47:1923–35.
Small SA. Isolating pathogenic mechanisms embedded within the hippocampal circuit through regional vulnerability. Neuron. 2014;84:32–9.
Lieberman JA, Small SA, Girgis RR. Early detection and preventive intervention in schizophrenia: from fantasy to reality. Am J Psychiat. 2019;176:794–810.
Acknowledgements
We would like to acknowledge the patients who participated in this study. We thank Xiaoyan Xu, Rawad Ayoub and Jiayan Meng for excellent technical support. The project described was supported by the Doris Duke Charitable Foundation as well as the Brain and Behavior Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RRG has recently received research support from Otsuka, Allergan, BioAdvantex and Genentech and has received advances/royalties from books published by Wipf and Stock and Routledge/Taylor and Francis. MS has consulted for Curasen Therapeutics. GB discloses that he receives royalties and/or advances from Routledge/Taylor and Francis and Prometheus Books. LSK has received research support from Amgen. JAL has received support administered through his institution in the form of funding or medication supplies for investigator-initiated research from Lilly, Denovo, Biomarin, Novartis, Taisho, Teva, Alkermes, and Boehringer Ingelheim, and is a member of the advisory board of Intracellular Therapies and Pierre Fabre. He neither accepts nor receives any personal financial remuneration for consulting, advisory board or research activities. He holds a patent from Repligen and receives royalty payments from SHRINKS: The Untold Story of Psychiatry. AAD reports serving on an advisory board for Sunovion, receiving an honorarium from Otsuka, receiving stock options from Terran Biosciences and System1 Biosciences, and receiving research support from Neurocrine and LB Pharmaceuticals in the previous year. No other authors report relevant conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Girgis, R.R., Slifstein, M., Brucato, G. et al. Imaging synaptic dopamine availability in individuals at clinical high-risk for psychosis: a [11C]-(+)-PHNO PET with methylphenidate challenge study. Mol Psychiatry 26, 2504–2513 (2021). https://doi.org/10.1038/s41380-020-00934-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-020-00934-w
This article is cited by
-
How changes in dopamine D2 receptor levels alter striatal circuit function and motivation
Molecular Psychiatry (2022)