Abstract
Ovarian carcinoma has the highest mortality of all female reproductive cancers and current treatment has become histotype-specific. Pathologists diagnose five common histotypes by microscopic examination, however, histotype determination is not straightforward, with only moderate interobserver agreement between general pathologists (Cohen’s kappa 0.54–0.67). We hypothesized that machine learning (ML)-based image classification models may be able to recognize ovarian carcinoma histotype sufficiently well that they could aid pathologists in diagnosis. We trained four different artificial intelligence (AI) algorithms based on deep convolutional neural networks to automatically classify hematoxylin and eosin-stained whole slide images. Performance was assessed through cross-validation on the training set (948 slides corresponding to 485 patients), and on an independent test set of 60 patients from another institution. The best-performing model achieved a diagnostic concordance of 81.38% (Cohen’s kappa of 0.7378) in our training set, and 80.97% concordance (Cohen’s kappa 0.7547) on the external dataset. Eight cases misclassified by ML in the external set were reviewed by two subspecialty pathologists blinded to the diagnoses, molecular and immunophenotype data, and ML-based predictions. Interestingly, in 4 of 8 cases from the external dataset, the expert review pathologists rendered diagnoses, based on blind review of the whole section slides classified by AI, that were in agreement with AI rather than the integrated reference diagnosis. The performance characteristics of our classifiers indicate potential for improved diagnostic performance if used as an adjunct to conventional histopathology.
Similar content being viewed by others
Introduction
Ovarian carcinoma is the deadliest cancer of the female reproductive system, with an estimated 13,770 deaths in the U.S. in 20211. It is also a heterogeneous disease with five common histotypes: high-grade serous carcinoma (HGSC) accounts for 70% of cases (and 90% of advanced-stage disease and mortality), clear cell ovarian carcinoma (CCOC) accounts for 12%, endometrioid (ENOC) for 11%, low-grade serous (LGSC) for 4%, and mucinous carcinoma (MUC) for 3%. These five common histotypes have distinct cellular morphologies and etiologies, as well as molecular, genetic, and clinical attributes2,3. There has been a move towards histotype-based treatment4, for example with the introduction of PARP inhibitor therapy for patients with HGSC5, and this trend increases the importance of accurate histotype diagnosis in practice6.
Ovarian carcinoma histotype classification by pathologists is associated with challenges in diagnostic reproducibility and interobserver disagreement4,7,8. Initial diagnosis is performed through histological assessment of hematoxylin & eosin (H&E)-stained sections, but studies have shown that for pathologists without current, gynecologic pathology-specific training, the interobserver agreement is only moderate (0.54–0.67 Cohen’s kappa9)10,11. Furthermore, the number of pathologists trained has not kept up with the increasing volume and complexity of cancer diagnoses. There has been a 4.5% increase per year in the amount of histopathology requests to laboratories in the United Kingdom, without a simultaneous increase in the pathology workforce. In fact, there is a projected pathologist shortage with >75% of full-time pathologists being 45 years or older and a relative decrease in the number of new pathology residents compared to other specialties12.
A potential way to enhance pathologist efficiency and diagnostic accuracy is to use machine learning as a diagnostic adjunct. In the past decade, machine learning models have demonstrated their potential for a wide range of applications in digital pathology, including cancer diagnoses13,14,15,16. Wang et al.17 introduced a two-stage transfer learning model for ovarian cancer classification that exceeded the Cohen’s kappa of general pathologists. However, they only trained and tested their model with 305 H&E slides from a single dataset. Differences between scanners, tissue processing, and staining procedures between laboratories contribute to substantial variation between datasets18,19,20; to truly serve as a diagnostic adjunct for practicing pathologists, a generalizable algorithm that can classify ovarian cancer cases from different centers is needed.
In this paper, aiming to develop a generalizable model for automatic ovarian carcinoma histotype classification, we used four different deep learning-based algorithms17,21,22 on a large dataset of 948 H&E-stained whole slide images (WSIs) and evaluated the performance on a held-out set of 60 cases from a different institution. To the best of our knowledge, our model achieved the highest performance for ovarian carcinoma histotype diagnosis based on an out-of-distribution test set to date and approached the performance level of expert gynecologic pathologists.
Materials and Methods
Data acquisition and processing
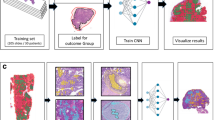
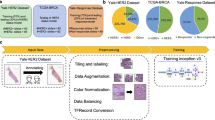
Two datasets from separate centers containing WSIs of the five common histotypes of ovarian carcinoma were used in this study. To prove the generalizability of our models, we used the first dataset for training and the second dataset (from a different hospital) was used for testing only. A visual summary of the procedure is shown in Fig. 1.
The first dataset (referred to henceforth as Internal Training Dataset) retrieved from the OVCARE archives consisted of 948 WSIs (scanned at 40× objective magnification on an IntelliSite Ultra-Fast Scanner (Philips, Amsterdam, Netherlands)) of 485 patients. The breakdown of the histotypes as shown in Table 1 is as follows: 410 HGSC slides (200 patients), 167 CCOC slides (95 patients), 237 ENOC slides (114 patients), 69 LGSC slides (34 patients), and 65 MUC slides (42 patients). The reference diagnosis for each patient was defined by combination of expert pathology review and molecular assays, typically IHC but also including sequencing in a subset of cases, to give an “integrated” diagnosis23. Using a combination of annotations from board-certified pathologists (for 416 slides) and pseudo annotations, a maximum of 150 patches per tumor and maximum of 20,000 patches per histotype were extracted from the tumor areas of all the slides at multiple sizes and magnifications (see Table 1). For example, to get 512 × 512 pixel patches at 20× magnification, patches of size 1024 × 1024 pixels at 40× magnification were down-sampled using the Lancoz filter24. The pseudo annotations were created using a stroma-tumor binary classifier trained on patches from the 416 slides with annotations (see Supplementary Information: Creating Pseudo Annotations). This stroma-tumor classifier has a mean area under receiver operating characteristic (ROC) curve (AUC) of 0.9441 (see Supplementary Table S1 and Supplementary Fig. S1) and was also shown to reliably filter non-malignant samples, such as benign ovarian tissue or benign fallopian tube cases (see Supplementary Tables S2, S3). We chose to limit the training dataset to 20,000 patches per histotype to create a partially balanced dataset because the least represented histotype (MUC) had approximately 10,000 patches extracted. Then each set of patches was grouped by patient origin into a 3-fold cross validation scheme for training (66%), validation (17%), and testing (17%).
The External Test Dataset comprised 60 WSIs (scanned at 40× magnification from an Aperio CSO scanner (Leica Biosystems, Buffalo Grove, IL, United States)) of 60 tumors (from 60 patients) from the University of Calgary. The slides consisted of 31 HGSC, 10 CCOC, 10 ENOC, 4 LGSC, and 5 MUC. The reference diagnoses for these cases was made by one of the authors (MK) and included histologic examination in addition to an 8-marker IHC panel (COSPv3) that predicts ovarian carcinoma histotype with 93% diagnostic concordance25. All WSIs were annotated by a pathologist, and 150 patches of size 1024 × 1024 pixels at 40×magnification were tiled from the tumor regions of the slides belonging to each tumor and down sampled to 512 × 512 pixels at 20× magnification, similar to the Internal Training Dataset. This dataset was used for testing purposes only.
Color normalization
Both datasets were then color normalized using the strategy described by Boschman et al.26. A representative reference image from the Internal Training Dataset was chosen (Supplementary Table S4), and then each patch was randomly normalized by either the Reinhard27, Vahadane28, or Macenko29 methods. The rationale is that if there is not a singular color normalization method that is ideal for all datasets or tasks, using a combination of them to normalize the images should make the images diverse enough to train a generalizable model, but similar enough to overcome the domain shift from having different colors from different datasets.
Training the deep learning-based histotype classifier
We compared four deep learning-based models (details outlined below) for ovarian carcinoma histotype classification. The models were initialized with ImageNet pre-trained weights, and then each were fine-tuned with the color-normalized patches from the Internal Training Dataset, using the ImageNet mean and standard deviation to normalize the RGB pixel values of each patch between −1 and 1.
After training, the performance of the models were compared using the testing set of the respective Internal Training Dataset cross-validation split and the out-of-distribution External Test Dataset. In cases where there was a discrepancy between the reference diagnosis and the histotype diagnosed by artificial intelligence, two of the study pathologists (NS and CBG) reviewed the WSIs blinded to either the reference diagnosis or the AI diagnosis, and without access to the immunostaining results of the COSPv3 8-marker panel.
The GPU hardware used was either a Quadro RTX 500 (Nvidia, Santa Clara, CA, United States) or a Tesla V100-SXM2-32GB (Nvidia) based on availability.
One-stage transfer learning
For the first model, we used a one-stage transfer learning (1STL) algorithm. We implemented the PyTorch30 VGG1931 model with a modified last layer for five-histotype classification. VGG19 is a popular convolutional neural network that uses smaller, typically 3 × 3, filters in order to create a deeper network. Training was done with the patches of size 512 × 512 pixels at 20× magnification using a batch size of 8 and AMSGrad optimization32 with 0.0002 learning rate. For each experiment, the model was fine-tuned for seven epochs, with the state having the best validation patch-level overall accuracy saved and used for testing.
Test slide-level results were calculated using majority vote from patch-level results (i.e., argmax on the counts of the different patch histotype predictions for each slide).
DeepMIL
The second architecture was DeepMIL22, a model that combines permutation-invariant multiple instance learning (MIL) with an attention-based neural network. MIL is a type of supervised learning where the labelled data (i.e., a WSI with a diagnosis) is broken up into a “bag of instances” which are considered to be weakly labelled (i.e., patches from the WSI, but each patch is not individually labelled). DeepMIL computes bag-level (WSI-level) features from attention-weighted patch instance feature vectors, and then classifies them with a fully connected layer. In our DeepMIL implementation, the patch feature vectors were extracted using the 1STL model trained on the same cross-validation split of 512 × 512 patches at 20× magnification, using patch-level balanced accuracy as the metric for saving the best model state. Then DeepMIL was trained for 300 epochs with an initial learning rate of 0.0001; the learning rate was decreased by half if the validation loss did not decrease for 15 consecutive epochs, and training was stopped if the validation loss did not decrease for 30 epochs. The metric used for saving the best model state for testing was the slide-level overall accuracy. The test set slide-level results were calculated directly using this model.
VarMIL
Thirdly, we implemented VarMIL21, a model based on DeepMIL. One limitation of DeepMIL is that the bag-level latent features based on the attention-weighted instance vectors do not consider tile interactions or high-level features of the bag (WSI). VarMIL extends the architecture with an additional attention-weighted variance module to represent the tissue heterogeneity of the different tiles in a WSI. Just as our implementation of DeepMIL, we used trained 1STL models for patch-level feature extraction. We then trained VarMIL with the 512 × 512 patches at 20× magnification and the same learning rate decay patience strategy as with DeepMIL. Slide-level overall accuracy was used to save the best state for testing. This model also calculated slide-level results directly.
Two-stage transfer learning
The final model we compared was a two-stage deep transfer learning (2STL) algorithm introduced for ovarian cancer histotype classification17. The first stage trains with patches of size 256 × 256 at 10× magnification, while the second stage training and testing uses patches of size 512 × 512 at 20× magnification. The rationale is that training with WSI patches of multiple sizes and magnifications gains context of the tissue at different perspectives. Each mini-batch is manipulated so that the same number of patches of each histotype is used during training. We used a batch size of 16 for the first stage and a batch size of 8 for the second stage. Each stage was trained with learning rate 0.0002 for 10 epochs, with the state with the highest patch-level overall balanced accuracy saved for the next stage of testing. The 3-fold cross validation scheme was modified by alternatively swapping the validation and test set for each training set, yielding a 6-fold scheme. The slide-level results were calculated by training a random forest classifier on the 6 patch-level cross-validation splits.
Results
To find the model architecture with the best performance, we compared four deep learning networks for ovarian carcinoma histotype classification: a one-stage transfer learning algorithm (1STL), DeepMIL, VarMIL, and a two-stage transfer learning algorithm (2STL). Each model was trained and tested on patches from the large Internal Training Dataset for three cross-validation splits. The three trained models from cross-validation splits were then tested on the External Test Dataset from a different center.
Due to the distribution shift that exists between H&E datasets from different centers, even of the same tissue type, we focus on the results on the External Test Dataset. In order to effectively supplement pathologists with a machine learning-based ovarian carcinoma classifier, the model must be generalizable enough to work on WSIs from various locations. Our criterion for choosing the best model was the highest mean slide-level diagnostic concordance on the External Test Dataset.
Our results show that the highest performing model was 1STL (Table 2), which achieved a mean slide-level diagnostic concordance of 80.97 ± 0.03 % on the External Test Dataset. This model was trained with color normalized patches from the Internal Training Dataset that were partially balanced across the histotypes. We performed additional experiments for all the models, including no color normalization and a larger, unbalanced set of patches (see Supplementary Table S5), and found that this strategy yielded the best results on the External Test Dataset (see Supplementary Tables S6–S17 and Supplementary Figs. S3–S6). For completeness, we also calculated slide-level results for 1STL with random forest classifiers and for 2STL with majority vote, but these results did not affect our conclusion (see Supplementary Tables S8, S9, S14, S15). As well, we tested this best-performing model on patches from the External Test Dataset using the pseudo annotation classifier rather than pathologist annotations and found the exact same ensemble classifier results (see Supplementary Table S18).
Table 3 shows the performance of the 1STL model across the three cross-validation splits for the Internal Training Dataset. Furthermore, it shows the results for the External Test Dataset, where the three models trained based on the cross-validation splits (as separate raters) were applied to the External Test Dataset. In addition, we formed an ensemble classifier in which the three models (i.e., raters) in a majority voting strategy predicted the histotype. Using this strategy, we achieved a Cohen’s kappa value of 0.77 in predicting histotypes which was better than the mean kappa value of 0.75 (Table 3).
Given that in clinical practice, multiple slides per tumor are examined to make a diagnosis, we asked whether our deep learning model would perform better when provided with multiple slides for a given tumor. Because we only had one slide per tumor in the External Dataset, we were only able to test this hypothesis in our Internal Training Dataset. Table 4 shows that the mean case-level concordance based on examination of multiple slides was higher than the slide-level results (86.56% (Table 4) versus 81.38% (Table 3)) when we used a majority voting strategy in which histotype was assigned based on the histotype diagnosis of the majority of slides.
Figure 2 shows the confusion matrix associated with the Ensemble Model (Table 3). We can see that the models generally struggled with classifying the ENOC slides of the External Test Dataset; even the ensemble classifier (Fig. 2) misdiagnosed half of the ENOC external test cases as HGSC or MUC. The 8 discrepant cases were independently reviewed by 2 of the authors (CBG, NS) blinded to the reference and AI diagnoses. Looking specifically at these 8 cases that were misclassified (Table 5 and Fig. 3), there are a variety of scenarios that could account for the misclassification. Cases A and D showed transitional pattern, an architecture that can be seen in either HGSC or ENOC, and IHC may be needed, as in these cases, for correct histotype diagnosis. Case B is a rare tumor in which the histotype is not clear, even after performing IHC, and arguably would best be classified as carcinoma NOS, as was done in the original cancer registry entry. Cases C, G, and H are examples of the differential diagnosis between MUC and ENOC; when there is depletion of intracellular mucin, or slides showing the borderline areas are not available for review, this differential diagnosis is indeed challenging and IHC may be needed. Of these 3 cases, Case C additionally demonstrates the difficulty in distinction between CCOC and ENOC; this case was morphologically considered to be CCOC on independent review, and the IHC results, principally PR-, would support this view, although the reference and cancer registry diagnoses are of ENOC. In Case F, the differential diagnosis rests between CCOC and HGSC with clear cell change, a diagnostic challenge that can be resolved with IHC. For these 7 cases, the independent pathologist review agreed with the AI diagnosis in 4 cases, highlighting how histotypes of ovarian carcinoma can exhibit morphological mimicry, showing features on H&E that mimic other histotypes with respect to architecture and cytological features, at least focally. Only case E was a clear error by AI classification, with two of three models diagnosing HGSC when it is a classic low-grade ENOC.
Figures A–H correspond to the 8 discordant cases from the external dataset that were misclassified by AI; details of these cases are listed in Table 5.
This phenomenon of morphological mimicry is further demonstrated in Fig. 4, which visually illustrates the best-performing 1STL model on patch-level data. The predicted class of each patch generally makes sense, even for the incorrectly predicted tiles. For example, in Fig. 4D, the chosen patch that was misclassified as MUC has a structure that could be misconstrued for mucin, the defining morphological feature of MUC.
We also tested the best performing 1STL model on 21 WSI samples of ovarian tumors that do not fall within the five common histotypes; although the prediction confidence was lower for these cases (see Supplementary Results and Supplementary Table S19), further work is needed to reliably detect and classify these other tumor types, however, this is outside the scope of the current investigation.
Discussion
Our main objective in this work was to elucidate a generalizable (i.e., applicable to slides prepared in different laboratories) machine learning-based strategy for improving ovarian carcinoma histotype diagnosis. We trained four different machine learning architectures with a variety of data engineering strategies and evaluated their performance on an external dataset. To the best of our knowledge, our training dataset of 948 WSIs is the largest collection of labelled ovarian carcinoma histotype images in published machine learning studies. Our chosen metric for comparing methods is average diagnostic concordance with expert integrated histotype diagnosis, based on consideration of both H&E morphology and IHC, using an external dataset from a different hospital to prove the generalizability of our results on an independent test set different from the training set.
Our proposed models achieved a mean slide-level diagnostic concordance of 80.97 ± 0.03% using a one-stage deep transfer learning network (1STL). Based on our training set results, we expect the patient-level concordance (if given a test set with multiple slides per patient, which is typical of clinical diagnostic work) to be higher than the slide-level concordance. Our 1STL model also out-performed our implementations of DeepMIL22, VarMIL21, and a two-stage deep transfer learning network, which was previously used for ovarian cancer classification on a single dataset, trained and tested on 305 WSIs17. When using such a large dataset for training, we found that balancing the histotypes helps to prevent overfitting on any overrepresented classes. We also found that color normalization is essential for making a model generalizable for H&E images processed at different hospitals. We used a color normalization strategy that utilizes multiple normalization methods to create sets of images that are similar enough but variable enough to make the network robust26, a promising strategy for overcoming the color inconsistencies of H&E images that has been a persistent problem for computer-aided diagnostics.
Wang et al. previously reported a high level of interobserver agreement using the two-stage transfer learning network, with better performance than general pathologists17. However, their ovarian cancer classifier performance was trained and tested on a single dataset of 305 WSIs. We have achieved an overall Cohen’s kappa of 0.7722 on a test set stained and processed in a completely different location than the training set; this exceeds the inter-rater reliability by general pathologists (0.54–0.67 kappa)10,11 and approaches the level of expert pathologists with gynecologic pathology training (0.73–0.97 kappa)23,33. Our generalizable strategy, which yields high performance on histopathology slides originating from other centers, is a further step towards the implementation of deep learning tools as a diagnostic adjunct for pathologists in diagnosing ovarian carcinoma histotype.
The “gold standard” for ovarian carcinoma histotype diagnosis is the integrated expert diagnosis, taking into account H&E morphology across all slides showing tumor, and using select molecular markers23. There remain challenges in histotype diagnosis, however, as there can be discrepancy between H&E morphology and molecular markers, as assessed by immunohistochemistry25. This is especially true for ENOC, where the differential diagnosis includes CCOC, MUC and HGSC25, and where IHC data may be necessary for accurate histotype diagnosis. It is encouraging that the challenges in histotype diagnosis by AI are identical to those encountered by expert pathologists. Indeed, in 4 of 8 cases from the external dataset, the expert review pathologists rendered diagnoses, based on blind review of the WSIs classified by AI, that were in agreement with AI rather than the integrated reference diagnosis. Based on this we believe that, opportunities for improvement notwithstanding, the diagnostic algorithm presented is ready for validation studies in clinical practice, performing at a level comparable to an expert gynecological pathologist in formulating a favored histotype diagnosis based on H&E morphology. It is important to note, however, that the algorithm will misclassify cases, and these are exactly those cases that surgical pathologists must be aware of and resort to IHC in order to accurately diagnose histotype, e.g., cases with unusual features where ENOC is in the differential diagnosis. This algorithm cannot replace the function of diagnostic surgical pathologists to take into account all information in a case, beyond that present on the H&E stained slide, but can formulate a favored diagnosis with a high degree of diagnostic concordance, within seconds. We envision that such a tool could be used routinely in the setting of a fully digital surgical pathology service, as a diagnostic adjunct.
In conclusion, we demonstrate a deep learning strategy for ovarian carcinoma histotype classification based only on histological features that is generalizable even on an externally stained test set. The performance is at a level that it could be implemented into practice, for validation. This approach holds potential as an adjunct for informing histotype diagnosis and in supporting histotype-specific ovarian cancer treatment.
Data availability
The color normalization and deep learning codebase developed for this study will be made available through the following address upon publication: https://github.com/AIMLab-UBC/.
References
Siegel, RL, Miller, KD, Fuchs, HE, Jemal, A. Cancer Statistics, 2021. CA Cancer J Clin 71, 7–33, https://doi.org/10.3322/CAAC.21654 (2021).
WHO Classification of Tumours Editorial Board. Female Genital Tumours. WHO Classification of Tumours (IARC Lyon, France, 2020).
Köbel, M, Kalloger, SE, Boyd, N, McKinney, S, Mehl, E, Palmer, C, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med 5, 1749–1760 (2008)
Gilks, CB, Oliva, E, Soslow, RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol 37, 874–881 (2013)
Coleman, RL, Fleming, GF, Brady, MF, Swisher, EM, Steffensen, KD, Friedlander, M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer. N. Engl J Med 381, 2403–2415 (2019)
Bartoletti, M, Musacchio, L, Giannone, G, Tuninetti, V, Bergamini, A, Scambia, G, et al. Emerging molecular alterations leading to histology-specific targeted therapies in ovarian cancer beyond PARP inhibitors. Cancer Treat Rev 101, 102298 (2021)
Han, G, Sidhu, D, Duggan, MA, Arseneau, J, Cesari, M, Clement, PB, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol 26, 1594–1604 (2013)
Clarke, BA, Gilks, CB Endometrial carcinoma: controversies in histopathological assessment of grade and tumour cell type. J Clin Pathol 63, 410–415 (2010)
Cohen, J A coefficient of agreement for nominal scales. Educ Psychol Meas 20, 37–46 (1960)
Patel, C, Harmon, B, Soslow, R, Garg, K, DeLair, D, Hwang, S, et al. Interobserver agreement in the diagnosis of ovarian carcinoma types: Impact of sub-specialization. Lab Investig 92, 292A-292A (2012)
Köbel, M, Kalloger, SE, Lee, S, Duggan, MA, Kelemen, LE, Prentice, L, et al. Biomarker-based ovarian carcinoma typing: A histologic investigation in the ovarian tumor tissue analysis consortium. Cancer Epidemiol Biomark Prev 22, 1677–1686 (2013)
Lujan, G, Quigley, JC, Hartman, D, Parwani, A, Roehmholdt, B, Meter, B Van, et al. Dissecting the business case for adoption and implementation of digital pathology: a white paper from the Digital Pathology Association. J Pathol Inf 12, 17 (2021)
Coudray, N, Ocampo, PS, Sakellaropoulos, T, Narula, N, Snuderl, M, Fenyö, D, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med 24, 1559–1567 (2018)
Farnell, DA, Huntsman, D, Bashashati, A The coming 15 years in gynaecological pathology: digitisation, artificial intelligence, and new technologies. Histopathology 76, 171–177 (2020)
Campanella, G, Hanna, MG, Geneslaw, L, Miraflor, A, Werneck Krauss Silva, V, Busam, KJ, et al. Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med 25, 1301–1309, (2019)
Bejnordi, BE, Veta, M, Van Diest, PJ, Van Ginneken, B, Karssemeijer, N, Litjens, G, et al. Diagnostic assessment of deep learning algorithms for detection of lymph node metastases in women with breast cancer. JAMA 318, 2199–2210 (2017)
Wang, Y, Farnell, D, Farahani, H, Nursey, M, Tessier-cloutier, B, Jones, SJM et al. Classification of epithelial ovarian carcinoma whole-slide pathology images using deep transfer learning. Med Imaging Deep Learn 2020, 3–7, https://doi.org/10.48550/arXiv.2005.10957 (2020).
Yagi, Y Color standardization and optimization in Whole Slide Imaging. Diagn Pathol 6, 1–12 (2011)
Bancroft JD, Gamble M. Theory and Practice of Histological Techniques (Elsevier Health Sciences, 2008)
Lyon, HO, De Leenheer, AP, Horobin, RW, Lambert, WE, Schulte, EKW, Van Liedekerke, B, et al. Standardization of reagents and methods used in cytological and histological practice with emphasis on dyes, stains, and chromogenic reagents. Histochem J 26, 533–544 (1994)
Schirris, Y, Gavves, E, Nederlof, I, Horlings, HM, Teuwen, J, DeepSMILE: Self-supervised heterogeneity-aware multiple instance learning for DNA damage response defect classification directly from H&E whole-slide images, arXiv preprint (2021), https://doi.org/10.48550/arXiv.2107.09405
Ilse, M, Tomczak, JM, Welling, M Attention-based deep multiple instance learning. 35th Int Conf Mach Learn 5, 3376–3391 (2018)
Peres, LC, Cushing-Haugen, KL, Anglesio, M, Wicklund, K, Bentley, R, Berchuck, A, et al. Histotype classification of ovarian carcinoma: A comparison of approaches. Gynecol Oncol 151, 53–60, (2018)
Turkowski K, Filters for common resampling tasks (Academic Press Professional, Inc., USA, 1990)
Köbel, M, Luo, L, Grevers, X, Lee, S, Brooks-Wilson, A, Gilks, CB, et al. Ovarian carcinoma histotype: strengths and limitations of integrating morphology with immunohistochemical predictions. Int J Gynecol Pathol 38, 353–362 (2019)
Boschman, J, Farahani, H, Darbandsari, A, Ahmadvand, P, Van Spankeren, A, Farnell, D, et al. The utility of color normalization for AI-based diagnosis of hematoxylin and eosin-stained pathology images. J Pathol 256, 15–24 (2021)
Reinhard, E, Adhikhmin, M, Gooch, B, Shirley, P Color transfer between images. IEEE Comput Graph Appl 21, 34–41 (2001)
Vahadane, A, Peng, T, Sethi, A, Albarqouni, S, Wang, L, Baust, M, et al. Structure-preserving color normalization and sparse stain separation for histological images. IEEE Trans Med imaging 35, 1962–1971 (2016)
Macenko M, Niethammer M, Marron JS, Borland D, Woosley JT, X Guan, et al. A method for normalizing histology slides for quantitative analysis. In 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro 1107–1110 (2009)
Paszke A, Gross S, Massa F, Lerer A, Bradbury J, Chanan G, et al. Pytorch: An imperative style, high-performance deep learning library. Adv Neural Inf Process Syst 32, 8024–8035 (2019)
He K, Zhang X, Ren S, Sun J, Deep residual learning for image recognition. In Proceedings of the IEEE Computer Society Conference on Computer Vision and Pattern Recognition 770–778 (2016)
Reddi, SJ, Kale, S, Kumar, S, On the Convergence of Adam and Beyond. arXiv preprint (2018), https://doi.org/10.48550/arXiv.1904.09237
Köbel, M, Kalloger, SE, Baker, PM, Ewanowich, CA, Arseneau, J, Zherebitskiy, V, et al. Diagnosis of ovarian carcinoma cell type is highly reproducible: a transcanadian study. Am J Surg Pathol 34, 984–993 (2010)
Acknowledgements
We gratefully acknowledge the support of Thomas Kryton, BFA, Media Producer for scanning and processing the External Test Dataset.
Funding
This work was supported by CIHR (No. 418734), NSERC (RGPIN-2019-04896), Cascadia Data Alliance, Michael Smith Foundation for Health Research Scholar Award, OVCARE Carraresi, and UBC ObGyn funds.
Author information
Authors and Affiliations
Contributions
AB, CBG, and NS conceived the study. JB, HF, and AB designed the machine learning models and experiments. JB, AD, and PA developed necessary computational and visualization tools. JB and AD performed experiments. JB, HF, DF, CBG, NS, and AB interpreted and analyzed the data. JB, HF, CBG, NS, and AB wrote the first draft of the manuscript. DF, AZ, SJ, MK, DH, CBG, and NS contributed to data collection, slide annotation, computational analysis, and infrastructure, or provided pathology expertise. All authors critically reviewed the manuscript for important intellectual content and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All experiments were conducted in accordance with the Declaration of Helsinki and the International Ethical Guidelines for Biomedical Research Involving Human Subjects. Anonymized archival tissue samples were retrieved from the pathology archive at the BC Cancer Ovarian Care Research Program (OVCARE), University of British Columbia, and Vancouver General Hospital and were digitized after approval by the institutional ethics board (REB# H18-03646).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Farahani, H., Boschman, J., Farnell, D. et al. Deep learning-based histotype diagnosis of ovarian carcinoma whole-slide pathology images. Mod Pathol 35, 1983–1990 (2022). https://doi.org/10.1038/s41379-022-01146-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01146-z
This article is cited by
-
TLOD: Innovative ovarian tumor detection for accurate multiclass classification and clinical application
Network Modeling Analysis in Health Informatics and Bioinformatics (2024)
-
OralEpitheliumDB: A Dataset for Oral Epithelial Dysplasia Image Segmentation and Classification
Journal of Imaging Informatics in Medicine (2024)
-
Evaluation of the prognostic potential of histopathological subtyping in high-grade serous ovarian carcinoma
Virchows Archiv (2024)
-
Learning to Generalize over Subpartitions for Heterogeneity-Aware Domain Adaptive Nuclei Segmentation
International Journal of Computer Vision (2024)
-
Artificial intelligence in ovarian cancer histopathology: a systematic review
npj Precision Oncology (2023)