Abstract
Extranodal extension (ENE) is a significant prognostic factor for human papilloma virus (HPV)-negative head and neck squamous cell carcinoma and is incorporated into AJCC 8th edition pN stage. It remains controversial whether ENE or the degree of ENE is prognostically relevant in HPV-positive oropharyngeal squamous cell carcinoma (OPSCC). A detailed clinicopathologic review was conducted in a large retrospective cohort of 232 surgically treated patients with HPV-positive OPSCC and nodal metastasis. Fifty-six patients (24%) had nodal metastasis with ENE. The median vertical extent of ENE was 2.9 mm (range 0.2–20.3 mm), and the median horizontal span of ENE was 2.5 mm (range: 0.3–14.0 mm). Comparing with patients without ENE, those with ENE were associated with a higher number of positive lymph nodes, lymphovascular invasion, perineural invasion, adjuvant chemotherapy, larger primary tumor size, and shorter follow up period. Patients with ENE had shortened overall survival (OS), disease specific survival (DSS), disease free survival (DFS), distant metastasis free survival (DMFS), and regional recurrence free survival (RRFS) on univariate survival analysis. The 5-year OS, DSS, and DFS were 95%, 97%, and 90% respectively for the group without ENE, and 64%, 71%, and 65% respectively for the group with ENE. On Multivariate survival analysis, the presence of ENE was an independent adverse prognostic factor for OS, DSS, and DFS. Additionally, major ENE defined as a vertical extent of ≥4 mm or irregular soft tissue deposit independently predicted shortened OS, DSS, and RFS. In conclusion, the presence of ENE, in particular major ENE, is an independent prognostic factor in HPV-positive OPSCC. Therefore, we propose to document the presence and extent of ENE for these tumors. Consideration may be given for AJCC 9th edition to include ENE into pN stage of HPV-positive OPSCC.
Similar content being viewed by others
Introduction
Extranodal extension (ENE), defined as breach of the lymph node capsule by metastatic tumor, is a notorious eventuality in head and neck squamous cell carcinoma (HNSCC) and often an indication justifying adjuvant radiation or chemoradiation therapy1. Many groups have identified ENE as one of the most important predictors for shortened survival and increased risk of recurrence and metastasis in HPV-negative HNSCC2,3,4,5,6. Nevertheless, the prognostic significance of ENE in HPV-positive oropharyngeal squamous cell carcinoma (OPSCC), a biologically distinct subset of HNSCC, characterized by non-keratinizing histopathology, oropharyngeal location, propensity for nodal metastasis, low mutation burden, and improved prognosis, is less established7. Multiple early retrospective studies with variable number of cases, including a meta-analysis published in 2016 encompassing 561 patients with HPV-positive OPSCC with nodal metastasis, failed to demonstrate any prognostic relevance of ENE3,4,8,9,10,11,12. However, several recent large-scale studies based on the National Cancer Database (NCDB, N ranges from 2663 to 8780)13,14,15,16,17,18 as well as a recent meta-analysis of 3603 patients19, have identified ENE as an independent adverse prognostic factor for overall survival (OS) and/or distant metastasis free survival (DMFS) in HPV-positive OPSCC. Such incertitude is reflected by the current 8th edition of American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system for HNSCC20. While the presence of any pathologic evidence of ENE results in upstages of nodal metastasis for patients with HPV-negative HNSCC, ENE does not impact the pathologic nodal staging (pN) in HPV-positive OPSCC.
The current AJCC/UICC staging manual20 and the College of American Pathologist (CAP) cancer reporting protocol21 include the extent of ENE, defined as the vertical span of the ENE from the lymph node capsule, as an optional reporting element for HNSCC, including HPV-positive OPSCC. For HPV-negative oral SCC, ENE can be further classified as macroscopic with an extent >2 mm and microscopic with an extent ≤2 mm20, and the presence of macroscopic ENE was found to be prognostically relevant in multivariate survival analysis5,22.
To date, only a few studies on HPV-positive OPSCC have included the extent of ENE11,12,16. However, some of these studies did not include a pathology review to assess ENE and none have documented the ENE extent as a continuous variable nor performed Receiver operating characteristics (ROC) analysis to determine the ideal cutoff value. Therefore, additional studies are needed to clarify the significance of ENE and its extent in HPV positive-OPSCC.
Toward this aim, we gathered this large retrospective cohort of 232 patients with surgically managed, treatment-naive, metastatic HPV-positive OPSCC to lymph nodes, and performed a detailed clinicopathologic review using ROC analysis to determine the significance of ENE and the best cutoff of its extent in predicting tumor behavior.
Material and methods
Study cohort
The study was approved by the Institutional Review Board (IRB) of Memorial Sloan Kettering Cancer Center (MSKCC, New York, NY, USA). The retrospective cohort included 232 surgically managed treatment-naive patients who underwent neck dissection between 1985 and 2019 for metastatic HPV-positive OPSCC to the cervical lymph nodes. Among them, 173 patients were operated at MSKCC, whereas the remaining 59 had surgery elsewhere with the pathology slides reviewed at MSKCC. Forty-four cases were included in a previous study9.
Exclusion criteria included: carcinoma with negative or unknown HPV status; neck metastasis from an unknown primary, tumors with prior neoadjuvant therapy; tumors in which the ENE status cannot be determined due to tissue fragmentation, and/or nodal metastases that were only sampled by core biopsies or fine needle aspiration.
Pathologic review
All histologic slides were reviewed by at least one head and neck pathologist (BX or NK) to collect detailed pathologic parameters. Subsequently, all tumors with ENE were re-reviewed at consensus conferences by three head and neck pathologists (BX, NK, and RG) to confirm the presence and extent of ENE. HPV status was confirmed in all cases by p16 immunoexpression using the cutoff of >70% nuclear and cytoplasmic staining of moderate or strong intensity21, and/or positivity for high-risk HPV RNA or DNA in situ hybridization (ISH) on either the primary or the metastatic tumor.
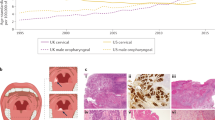
ENE was defined as the extension of metastatic tumor through the lymph node capsule into the perinodal fibroadipose tissue and/or skeletal muscle. Soft tissue deposit was defined as metastasis without any associated lymph node structure (germinal centers or subcapsular sinus). Soft tissue deposit was further divided into two subcategories: rounded/well-circumscribed with a well-defined smooth border; and infiltrative/irregular with irregular and infiltrative outer contour (Fig. 1). Rounded soft tissue deposit was considered as nodal metastasis without ENE, whereas irregular soft tissue deposit was regarded as nodal metastasis with major ENE in which the exact extent of ENE could not be measured as the deposit did not have any identifiable lymph node capsule.
A, B The vertical extent of ENE is measured from the external boundary of the lymph node capsule perpendicularly to the most distantly located tumor clusters. A Major ENE has a vertical extent of ≥4 mm. B Minor ENE has a vertical extent of <4 mm. Insert: Tumor (T) breaches the lymph node capsule (arrowheads) and invade into perinodal fibroadipose tissue. C A regular soft tissue deposit is a rounded/well-circumscribed soft tissue deposit without recognizable lymph node architecture (considered as nodal metastasis without ENE). D An irregular soft tissue deposit is an infiltrative soft tissue deposit without recognizable lymph node architecture (considered as major ENE in which the extent of ENE cannot be measured).
The vertical span of ENE was measured from the recognizable outer boundary of the lymph node capsule perpendicularly to the most distal perinodal tumor clusters (Fig. 1). The horizontal span of ENE was measured parallel to the recognizable lymph node capsule. Other parameters of ENE reviewed and recorded included: number of lymph node with ENE, size of the lymph node with the most ENE, ratio of the size of metastasis to the lymph node size in the lymph node with the most ENE, tissue involved by ENE (being fibroadipose tissue vs. skeletal muscle), and desmoplastic reaction within ENE foci.
Other characteristics of the primary and metastatic tumors that were collected included: total number of lymph nodes sampled, total number of lymph nodes positive for metastasis; size of the largest positive lymph node and largest metastatic focus, laterality of the positive lymph node (ipsilateral vs. bilateral), primary tumor size, lymphovascular invasion, perineural invasion, margin status, and AJCC 8th edition pT and pN stage.
Clinical review and outcomes
Clinical parameters, such as age, sex, post-operative radiation therapy, post-operative chemotherapy, and outcome data were collected. Follow up data were available in 220 patients and were calculated from the day of primary resection. Outcome assessment included OS, DSS, recurrence free survival (RFS), DMFS, local recurrence free survival (LRFS), and regional recurrence free survival (RRFS).
Statistics
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, Armonk, NY, U.S.). Comparisons of clinicopathologic features between tumors with and without ENE were performed using Chi square test or Fisher’s exact test for categorical variables and two-tailed Student’s t test for continuous variables. Univariate survival analysis was conducted using log rank test for categorical variables and Cox hazards proportional model for continuous variables. Variables significant on univariate analysis were subjected to multivariate survival analysis using Cox Hazards proportional model. Cumulative 5-year, 10-year, and 15-year survival was calculated. To determine the best cutoff to separate major and minor ENE, ROC analysis was performed to evaluate the performance extent of ENE (vertical or horizontal) in predicting outcome.
Results
Clinicopathologic features
The clinicopathologic characteristics of the study cohort substratified by the ENE status are summarized in Table 1, and the characteristics of ENE are provided in Table 2. The median age of patients at time of diagnosis was 58 years (range: 34–88). The male to female ratio was 3.9:1. Grossly involved lymph nodes were typically representatively sampled. The median number of blocks from the largest metastatic lymph node was 2 (range: 1–13). The median number of blocks per cm from the largest positive lymph node was 0.55 (ranges: 0.14–2.82). The mean size of largest involved lymph node and largest metastatic focus was 3.3 cm and 3.1 cm respectively.
Among the 232 patients with metastatic HPV-positive OPSCC to the cervical lymph node(s), 56 patients (24%) had ENE. Compared with tumors without ENE, those with ENE were associated with significantly higher number of positive lymph nodes (p < 0.001), higher AJCC pN stage (p < 0.001), larger primary tumor size (p = 0.003), higher rate of lymphovascular invasion (p = 0.037), higher rate of perineural invasion (p = 0.003), shorter follow up period (p = 0.031) and a higher rate of adjuvant chemotherapy (p = 0.031). Other clinical and pathologic parameters, such as age, sex, number of resected lymph nodes, margin status, AJCC pT stage, and post-operative adjuvant radiation therapy, did not differ between the two groups.
Defining major and minor ENE
On ROC analysis, there was a significance correlation between the vertical span of ENE and DSS (area under curve AUC = 0.703, p = 0.048). The best threshold of vertical extent of ENE was determined at 4 mm. The horizontal span of ENE did not predict outcomes.
Given the results of ROC analysis, major ENE was defined as a vertical ENE extent of ≥4 mm. The presence of irregular soft tissue deposit was also considered as major ENE in which the extent of ENE cannot be measured.
Using these criteria, 21 of the 56 cases with ENE (37.5%) had major ENE, including 13 cases with irregular soft tissue deposits and 8 cases with a vertical ENE extent of ≥4 mm. The remaining 35 patients (62.5%) had minor ENE.
Prognostic significance of ENE
Follow up data were available in 220 patients with a median follow up period of 45 months (range 3–291 months). The observed events included 17 patients with disease-related mortality, and 29 patients with recurrences, including 16 with distant metastasis, 10 with local recurrence, and/or 12 with regional recurrence.
Log rank test showed that patients with ENE were associated with significantly shortened OS, DSS, RFS, DMFS, and RRFS compared with patients without ENE (Table 3 and Fig. 2). LRFS did not differ between the two groups. The cumulative 5-year and 10-year DSS was 97% and 95% respectively for the group without ENE, and 71% and 71% respectively with ENE. The cumulative 5-year and 10-year RFS were 90% and 81%, respectively for patients without ENE, and 65% and 65%, respectively for those with ENE.
Among the 173 patients who were operated at MSKCC, 171 patients had follow up data available. In this subgroup of patients, ENE remained a significant adverse prognostic factor for OS (p < 0.001), DSS (p < 0.001), and RFS (p < 0.001) on univariate survival analysis by log rank test.
When ENE was further divided into minor and major ENE according to the criteria defined above, minor ENE subgroup was shown to be associated with significantly shortened OS, DSS, DFS, and RRFS compared with the subgroup without ENE (log rank pairwise test, p < 0.05); whereas major ENE correlated with shortened OS, DSS, DFS, and DMFS compared with the subgroup with minor ENE (Table 3 and Fig. 2). The 5-year and 10-year DSS was 87% and 87% respectively in patients with minor ENE; and 48% and 48% respectively in patients with major ENE.
Two empirical cutoffs were additionally tested: one is the 2 mm cutoff included in AJCC 8th edition20; the other is the 1 mm cutoff used for Eastern cooperative oncology group (ECOG) E3311 trial23. Macroscopic ENE was defined as vertical ENE extent of >2 mm or >1 mm, or irregular soft tissue deposit, whereas microscopic ENE was defined as a vertical ENE extent of ≤2 mm or ≤1 mm. Using either threshold, there was no significant survival difference between the groups with macroscopic ENE and that with microscopic ENE on univariate log rank survival analysis (Table 4).
The prognostic significances of other clinicopathologic parameters on survival using univariate log rank test or Cox proportional hazards model are shown in Table 5. Factors predicting OS, DSS, and RFS included post-operative chemotherapy, tumor size, perineural invasion, AJCC 8th edition pT stage, number of positive lymph nodes, type of tissue involved by ENE, and irregular soft tissue deposits. Additionally, older age was associated with decreased OS; advanced AJCC pT stage was associated with decreased DSS and RFS; bilateral lymph node involvement was associated with decreased RFS; and increased number of lymph nodes with ENE was associated with decreased OS and DSS.
The results of multivariate survival analysis are shown in Table 6. The presence of ENE was a significant independent prognostic factor for shortened OS (hazard ratio HR = 3.220, 95% confidence interval CI = 1.301–7.971, P = 0.011), DSS (HR = 6.260, 95% CI = 1.593–24.597, P = 0.009), and DFS (HR = 3.153, 95% CI = 0.912–1.725, p = 0.026). Additionally, compared with patients without ENE, those with major ENE had decreased OS (HR = 3.739, 95% CI = 1.351–10.345, P = 0.011), DSS (HR = 11.488, 95% CI = 2.543–51.889, P = 0.002), and RFS (HR = 5.700, 95% CI = 1.715–18.938, P = 0.004). No significant difference was detected between the subgroup with minor ENE and that without ENE.
Other independent adverse prognostic factors included perineural invasion and high AJCC pN stage for OS; and laterality of involved lymph nodes for DFS.
Discussion
The reported rate of extranodal extension in HPV-positive OPSCC varies drastically across studies, ranging from as low as 10% to as high as 80%4,9,10,23,24,25,26,27. Such a wide range may be attributed to selection criteria for surgical management of HPV-positive OPSCC across various institutions. Studies based on NCDB data have reported a frequency of ENE of 32% to 44% in HPV-positive OPSCC14,15,16,17,18,19,28,29. In the current study, ENE was identified in 24% of the cases. Multiple factors may attribute to the large variation of reported ENE rate in HPV-positive OPSCC, including the lack of universally accepted definition of ENE in HNSCC, the presence of inter- and intra-observer discrepancy in interpretating ENE, and the variation of nodal sampling across different institutions. Indeed, the definition of ENE in HNSCC varies across different studies, ranging from any breach of the lymph node capsule by the metastatic tumor, to involvement of perinodal soft tissue with or without desmoplasia, to requiring a desmoplastic reaction of the metastatic tumor in the perinodal tissue30.
Not surprisingly, interobserver and intraobserver agreement among pathologists to evaluate the presence of ENE in HNSCC was reported to be weak to moderate at best with a kappa value ranging from 0.14 to 0.7531. Furthermore, the nodal metastasis of HPV-positive OPSCC is typically large and grossly evident. The median size of largest nodal metastasis was 3.0 cm (up to 7.0 cm) in the present study. Currently, there is no standardized gross assessment for cervical lymph nodes dissection. Sampling protocol for a grossly positive lymph node seems to be inconsistent and varies across institutions. In fact, the number of tumor blocks sampled from the largest metastatic lymph node in the current study showed a wide range from 1 section to 13 sections. Theoretically a low sampling rate may result in under-detection of ENE. Based on these data, considerations should be given to standardize the macroscopic examination protocol for cervical lymph nodes and to propose an internationally standardized histologic diagnostic criteria to evaluate the presence and the extent of ENE for HNSCC, including HPV-positive OPSCC.
The focus of metastatic HPV-positive OPSCC and the involved lymph node tend to be large. The mean size of the largest involved lymph node and the largest metastatic focus were 3.3 cm and 3.1 cm, respectively in our cohort. In contrast, the mean size of largest metastatic node and the largest metastatic focus in HPV-negative oral squamous cell carcinoma were 1.6 cm and 1.0 cm, respectively5. In many cases, the positive node was almost completely replaced by tumor. Lobulation and fibrous septation were noted in a proportion of cases, which may make the assessment of ENE more challenging and variable among observers.
Such a wide variation in sampling and reporting ENE may also be an important explanation of the conflicting literature regarding the prognostic significance of ENE in HPV-positive OPSCC. Multiple early retrospective studies with a cohort size of 76–210 HPV-positive OPSCC, including the earlier study from our institution9 reported that the presence of ENE did not predict survival and recurrence3,9,10,11,12,24. A meta-analysis published in 2016 including a total of 561 patients with HPV positive- OPSCC metastatic to lymph node from four retrospective studies published between 2012 and 2016 showed that pathologic ENE did not significantly impact DFS with a HR of 1.39 (95% CI: 0.12–15.81)4. On the other hand, several more recent retrospective studies with a cohort size ranging from 74 to 296 concluded that the presence of ENE was an adverse prognostic factor for OS, DSS, and RFS on univariate8,25,27,32 and/or multivariate survival analysis33. Moreover, a recent meta-analysis including 3603 patients with HPV-positive OPSCC from 18 retrospective studies shown that pathologic ENE was an independent adverse prognostic factor associated with decreased OS (HR = 1.89, 95% CI: 1.15–3.13), and distant metastasis free survival (HR = 3.23, 95% CI: 1.25–8.33), but not locoregional recurrence and DSS19. Additionally, multiple studies using NCDB data with slightly different patient selection criteria including 2663–8780 patients with HPV-positive OPSCC universally demonstrated that ENE independently predicted decreased OS3,4,8,9,10,11,12. In the current study, we conducted a rigorous pathology review to evaluate the presence, extent, and associated features of ENE. We have found that the presence of pathologic ENE was an independent adverse pathologic feature for decreased OS, DSS, and DFS, when adjusted for other prognostic features, such as age, tumor size, AJCC pN stage, and perineural invasion. Taken together, an overwhelming body of literature including large-scale studies has indicated an important prognostic role of ENE in HPV-positive OPSCC. Therefore, pathologic ENE should be routinely evaluated and documented in the pathology report of surgically treated HPV-positive OPSCC.
Furthermore, the current study aimed to assess the prognostic significance of ENE extent and soft issue tumor deposit in HPV-positive OPSCC, which was addressed only in a few prior studies. The vertical span of ENE is typically measured as a continuous variable from the recognizable outer contour of an involved lymph node to the more distant aspect of perinodal invasion. Besides, ENE can be classified into macroscopic (major) and microscopic (minor) using ROC analysis to determine the most prognostically relevant cutoff value of the ENE extent5. In patients with HPV-negative oral SCC, the ideal cutoff of ENE determined using ROC analysis was reported as 1.7 mm in the study by Wreesmann et al.5, and 1.9 mm in the study by Mamic et al.22. Macroscopic ENE was found to be associated with decreased survival and increased risk of recurrence, while minor ENE had no significant impact on survival5,22. Based on these studies, the 8th edition of AJCC/UICC staging has adopted 2 mm as the cutoff to separate macroscopic and microscopic ENE. The current study was the first to perform ROC analysis to assess the significance of ENE extent in HPV-positive OPSCC. We have found the best cutoff to define major (macroscopic) ENE to be 4 mm. Using this cutoff, major ENE was associated with a significantly decreased OS (HR = 3.739), DSS (HR = 11.488), and DFS (HR = 5.700) compared with nodal metastasis without ENE on multivariate analysis. On the other hand, minor ENE was only associated with a shortened OS, DSS, and DFS on univariate but not multivariate analysis. The 5-year disease specific survival decreased from 97% in patients with HPV-positive OPSCC without ENE, to 87% in those with minor ENE, to 48% in those with major ENE. Therefore, it is crucial for pathologists to document not only the presence but also the extent of ENE in HPV-positive OPSCC as both parameters serve as independent prognostic factors to allow risk-stratification and planning for appropriate adjuvant therapy.
Interestingly, the performance of 2 mm cutoff currently included in AJCC 8th staging manual and 1 mm cutoff used in ECOG E8811 trial23 appear to be less than ideal in HPV-positive OPSCC compared with the 4 mm threshold determined using ROC analysis. There is a significant difference of OS, DSS, and DFS among all three groups (no ENE, minor ENE, and major ENE) using 4 mm as a cutoff for major ENE (Table 3), whereas no significant survival difference is detected between macroscopic and microscopic ENE using 2 mm or 1 mm cutoffs, respectively (Table 4). These data support the fact that HPV-positive OPSCC is biologically different from HPV-negative HNSCC, and 4 mm appear to be a better cutoff value to stratify ENE in HPV-positive OPSCC. Regardless, we believe that it is important for pathologists to report the exact extent of ENE in millimeter as a continuous variable to collect sufficient data for future studies to determine the significance and ideal thresholds for ENE in HNSCC.
To date, only three studies have included measurement on ENE extent in HPV-positive OPSCC. Two retrospective studies of OPSCC from Washington University, including a subset of p16-positive cancers (n = 152 and n = 90 respectively) and using an arbitrary ENE extent cutoff of 1 mm have found that the presence and the extent of ENE did not alter OS, DSS, or DFS in OPSCC11,12. Using NCDB data, Bauer et al. recently reported that both microscopic ENE (n = 516) and macroscopic ENE (n = 133), as defined by the AJCC 8th edition were associated with decreased OS compared with those without ENE in surgically managed HPV-positive OPSCC16. All three studies have certain weaknesses: the first two did not perform subgroup analysis based on HPV status, whereas the third was a database study without pathology review. Neither study documented the ENE extent as a continuous variable nor performed ROC analysis to determine the ideal cutoff value.
Two studies from Washington University have shown that irregular soft tissue deposit, defined as metastatic focus in the soft tissue without residual nodal tissue architectures (such germinal center or subcapsular sinus), was associated with decreased OS, DSS and DFS on univariate11,12 and multivariate survival analyses11 in HPV-positive OPSCC. Similarly, we have found that irregular soft tissue deposit was associated with decreased OS, DSS, and RFS on univariate analysis. Together, these data support the notion that irregular soft tissue deposit should be regarded as macroscopic (major) ENE, although the exact extent of ENE cannot be determined due to the absence of an appreciable lymph node capsule.
In conclusion, pathologic ENE, defined as metastasis breaching the lymph node capsule and invading the perinodal soft tissue, is an independent adverse prognostic factor associated with decreased OS, DSS, and DFS in surgically treated HPV-positive OPSCC with cervical nodal metastasis. In addition, further characterization of ENE extent into major ENE (defined as vertical ENE span of ≥4 mm and/or irregular soft tissue deposit) and minor ENE (defined as vertical ENE span of <4 mm) provides additional prognostic information in HPV-positive OPSCC. Consideration should be given for the pathologists to routinely document the presence and extent of ENE, and for ENE to be adopted into the next AJCC staging manual for the pathologic nodal staging of HPV-positive OPSCC.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Pfister, D. G., Spencer, S., Adelstein, D., Adkins, D., Brizel, D. M., Bruce, J. Y. et al. NCCN clinical practice guidelines in oncology: head and neck cancers. Version 3.2021, https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck_blocks.pdf Accessed 12/21/2021, (2021).
Puri, S. K., Fan, C. Y. & Hanna, E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr Opin Otolaryngol Head Neck Surg 11, 119–123 (2003).
Maxwell, J. H., Ferris, R. L., Gooding, W., Cunningham, D., Mehta, V., Kim, S. et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer 119, 3302–3308 (2013).
Mermod, M., Tolstonog, G., Simon, C. & Monnier, Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol 62, 60–71 (2016).
Wreesmann, V. B., Katabi, N., Palmer, F. L., Montero, P. H., Migliacci, J. C., Gonen, M. et al. Influence of extracapsular nodal spread extent on prognosis of oral squamous cell carcinoma. Head & neck 38 Suppl 1, E1192–1199 (2016).
Huang, S. H., Chernock, R., O’Sullivan, B. & Fakhry, C. Assessment Criteria and Clinical Implications of Extranodal Extension in Head and Neck Cancer. Am Soc Clin Oncol Educ Book 41, 265–278 (2021).
Saliba, M., Ghossein, R. & Xu, B. HPV-related head and neck cancers: Pathology and biology. Journal of surgical oncology 124, 923–930 (2021).
Kumar, B., Cipolla, M. J., Old, M. O., Brown, N. V., Kang, S. Y., Dziegielewski, P. T. et al. Surgical management of oropharyngeal squamous cell carcinoma: Survival and functional outcomes. Head & neck 38 Suppl 1, E1794–1802 (2016).
Iyer, N. G., Dogan, S., Palmer, F., Rahmati, R., Nixon, I. J., Lee, N. et al. Detailed Analysis of Clinicopathologic Factors Demonstrate Distinct Difference in Outcome and Prognostic Factors Between Surgically Treated HPV-Positive and Negative Oropharyngeal Cancer. Ann Surg Oncol 22, 4411–4421 (2015).
Klozar, J., Koslabova, E., Kratochvil, V., Salakova, M. & Tachezy, R. Nodal status is not a prognostic factor in patients with HPV-positive oral/oropharyngeal tumors. J Surg Oncol. 107, 625–633 (2013).
Sinha, P., Lewis, J. S., Jr., Piccirillo, J. F., Kallogjeri, D. & Haughey, B. H. Extracapsular spread and adjuvant therapy in human papillomavirus-related, p16-positive oropharyngeal carcinoma. Cancer 118, 3519–3530 (2012).
Lewis, J. S., Jr., Carpenter, D. H., Thorstad, W. L., Zhang, Q. & Haughey, B. H. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod Pathol, 24, 1413–1420 (2011).
Ho, A. S., Luu, M., Kim, S., Tighiouart, M., Mita, A. C., Scher, K. S. et al. Nodal staging convergence for HPV- and HPV+ oropharyngeal carcinoma. Cancer 127, 1590–1597 (2021).
Gal, T. J., O’Brien, K. J., Chen, Q. & Huang, B. Clinical vs Microscopic Extranodal Extension and Survival in Oropharyngeal Carcinoma in the Human Papillomavirus Era. Otolaryngol Head Neck Surg. 162, 693–701 (2020).
Day, A. T., Yang, A. M., Tanamal, P., Blackwell, J. M., Wang, E., Sumer, B. D. et al. Extracapsular extension, pathologic node status, and adjuvant treatment in primary surgery patients with human papillomavirus-mediated oropharyngeal cancer: National hospital-based retrospective cohort analysis. Head & neck 43, 3345–3363 (2021).
Bauer, E., Mazul, A., Chernock, R., Rich, J., Jackson, R. S., Paniello, R. et al. Extranodal extension is a strong prognosticator in HPV-positive oropharyngeal squamous cell carcinoma. Laryngoscope 130, 939–945 (2020).
Miccio, J. A., Verma, V., Kelly, J., Kann, B. H., An, Y., Park, H. S. et al. Impact of contralateral lymph nodal involvement and extranodal extension on survival of surgically managed HPV-positive oropharyngeal cancer staged with the AJCC eighth edition. Oral Oncol 99, 104447 (2019).
Zhan, K. Y., Eskander, A., Kang, S. Y., Old, M. O., Ozer, E., Agrawal, A. A. et al. Appraisal of the AJCC 8th edition pathologic staging modifications for HPV-positive oropharyngeal cancer, a study of the National Cancer Data Base. Oral Oncol 73, 152–159 (2017).
Benchetrit, L., Torabi, S. J., Givi, B., Haughey, B. & Judson, B. L. Prognostic Significance of Extranodal Extension in HPV-Mediated Oropharyngeal Carcinoma: A Systematic Review and Meta-analysis. Otolaryngol Head Neck Surg. 164, 720–732 (2021).
Amin, M. B., Greene, F. L., Edge, S. B.,Compton, C.C., Greshenwald, J. E., Brookland, R. K. et al. AJCC Cancer Staging Manual. Eighth edition., (Springer Nature: New York, 2017).
Seethala, R. R., Weinreb, I., Bullock, M. J., Calson, D. L., Ferris, R. L., Harrison, L. B. et al. Protocol for the Examination of Specimens From Patients With Cancers of the Pharynx https://documents.cap.org/protocols/HN.Pharynx_4.1.1.0.REL_CAPCP.pdf Accessed 12/21/2021, (2021).
Mamic, M., Lucijanic, M., Manojlovic, L., Muller, D., Suton, P. & Luksic, I. Prognostic significance of extranodal extension in oral cavity squamous cell carcinoma with occult neck metastases. Int J Oral Maxillofac Surg 50, 309–315 (2021).
Ferris, R. L., Flamand, Y., Weinstein, G. S., Li, S., Quon, H., Mehra, R. et al. Transoral robotic surgical resection followed by randomization to low- or standard-dose IMRT in resectable p16+ locally advanced oropharynx cancer: A trial of the ECOG-ACRIN Cancer Research Group (E3311). Journal of Clinical Oncology 38, 6500–6500 (2020).
Sinha, P., Kallogjeri, D., Gay, H., Thorstad, W. L., Lewis, J. S., Jr., Chernock, R. et al. High metastatic node number, not extracapsular spread or N-classification is a node-related prognosticator in transorally-resected, neck-dissected p16-positive oropharynx cancer. Oral Oncol 51, 514–520 (2015).
Kompelli, A. R., Morgan, P., Li, H., Harris, W., Day, T. A. & Neskey, D. M. Prognostic Impact of High-Risk Pathologic Features in HPV-Related Oropharyngeal Squamous Cell Carcinoma and Tobacco Use. Otolaryngol Head Neck Surg. 160, 855–861 (2019).
Kowalchuk, R. O., Van Abel, K. M., Yin, L. X., Garcia, J., Harmsen, W. S., Moore, E. J. et al. Correlation between radiographic and pathologic lymph node involvement and extranodal extension via CT and PET in HPV-associated oropharyngeal cancer. Oral Oncol 123, 105625 (2021).
Zebolsky, A. L., George, E., Gulati, A., Wai, K. C., Carpenter, P., Van Zante, A. et al. Risk of Pathologic Extranodal Extension and Other Adverse Features After Transoral Robotic Surgery in Patients With HPV-Positive Oropharynx Cancer. JAMA Otolaryngol Head Neck Surg. 147, 1080–1088 (2021).
Hararah, M. K., Stokes, W. A., Jones, B. L., Oweida, A., Ding, D., McDermott, J. et al. Nomogram for preoperative prediction of nodal extracapsular extension or positive surgical margins in oropharyngeal squamous cell carcinoma. Oral Oncol 83, 73–80 (2018).
An, Y., Park, H. S., Kelly, J. R., Stahl, J. M., Yarbrough, W. G., Burtness, B. A. et al. The prognostic value of extranodal extension in human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 123, 2762–2772 (2017).
Abdel-Halim, C. N., Rosenberg, T., Larsen, S. R., Høilund-Carlsen, P. F., Sørensen, J. A., Rohde, M. et al. Histopathological Definitions of Extranodal Extension: A Systematic Review. Head Neck Pathol 15, 599–607 (2021).
van den Brekel, M. W., Lodder, W. L., Stel, H. V., Bloemena, E., Leemans, C. R. & van der Waal, I. Observer variation in the histopathologic assessment of extranodal tumor spread in lymph node metastases in the neck. Head & neck 34, 840–845 (2012).
Waltonen, J. D., Thomas, S. G., Russell, G. B. & Sullivan, C. A. Oropharyngeal Carcinoma Treated with Surgery Alone: Outcomes and Predictors of Failure. Ann Otol Rhino Laryngol. https://doi.org/10.1177/00034894211021287, 34894211021287 (2021).
Freitag, J., Wald, T., Kuhnt, T., Gradistanac, T., Kolb, M., Dietz, A. et al. Extracapsular extension of neck nodes and absence of human papillomavirus 16-DNA are predictors of impaired survival in p16-positive oropharyngeal squamous cell carcinoma. Cancer 126, 1856–1872 (2020).
Funding
Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748.
Author information
Authors and Affiliations
Contributions
Study design and conception; BX, NK. Pathology and clinical review: BX, MS, BA, SD, RAG, NK. Database management and statistics: BX. Manuscript drafting: BX. Manuscript editing: MS, BA, MA, NL, NR, SGP, IG, SD, RAG, NK.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The study was approved by the institutional review board of MSKCC. All of the research meets the ethics guidelines, including adherence to the legal requirements of the country where the study was performed.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, B., Saliba, M., Alzumaili, B. et al. Prognostic impact of extranodal extension (ENE) in surgically managed treatment-naive HPV-positive oropharyngeal squamous cell carcinoma with nodal metastasis. Mod Pathol 35, 1578–1586 (2022). https://doi.org/10.1038/s41379-022-01120-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-022-01120-9
This article is cited by
-
The prognostic effect of radiological extranodal extension in HPV-positive oropharyngeal squamous cell carcinomas: a retrospective cohort analysis
European Archives of Oto-Rhino-Laryngology (2024)
-
Real world practice of postoperative radiotherapy for patients with completely resected pIIIA-N2 non-small cell lung cancer: a national survey of radiation oncologists in China
Radiation Oncology (2023)
-
CT radiomic signature predicts survival and chemotherapy benefit in stage I and II HPV-associated oropharyngeal carcinoma
npj Precision Oncology (2023)