Abstract
The morphologic spectrum of type 1 papillary renal cell carcinoma (PRCC) is not well-defined, since a significant proportion of cases have mixed type 1 and 2 histology. We analyzed 199 cases of PRCC with any (even if focal) type 1 features, with a median follow-up of 12 years, to identify clinicopathological features associated with outcome. Ninety-five tumors (48%) of the cohort contained some type 2 component (median amount: 25%; IQR: 10%, 70%). As a group they showed high rates of progression-free (PFS) and cancer-specific survival (CSS). Tumor size, mitotic rate, lymphovascular invasion, sarcomatoid differentiation, sheet-like architecture, and lack of tumor circumscription were significantly associated with CSS (p ≤ 0.015) on univariate analysis. While predominant WHO/ISUP nucleolar grade was associated with PFS (p = 0.013) and CSS (p = 0.030), the presence of non-predominant (<50%) nucleolar grade did not show association with outcome (p = 0.7). PFS and CSS showed no significant association with the presence or the amount of type 2 morphology. We compared the molecular alterations in paired type 1 and type 2 areas in a subset of 22 cases with mixed type 1 and 2 features and identified 12 recurrently mutated genes including TERT, ARID1A, KDM6A, KMT2D, NFE2L2, MET, APC, and TP53. Among 78 detected somatic mutations, 61 (78%) were shared between the paired type 1 and type 2 areas. Copy number alterations, including chromosome 7 and 17 gains, were similar between type 1 and 2 areas. These findings support that type 2 features in a PRCC with mixed histology represent either morphologic variance or clonal evolution. Our study underscores the notion that PRCC with any classic type 1 regions is best considered as type 1 PRCC and assigned the appropriate WHO/ISUP nucleolar grade. It provides additional evidence that type 2 PRCC as a separate category should be re-assessed and likely needs to be abandoned.
Similar content being viewed by others
Introduction
Papillary renal cell carcinoma (PRCC), accounting for approximately 15% of renal epithelial malignancy, was first described as a distinct subtype of renal cell carcinoma (RCC) in 1976 by Mancilla-Jiminez et al.1. In this study, the authors reported lower pathological stages and better 5-year-survival for PRCC compared to non-PRCC. These observations were validated in further studies2,3,4,5,6,7,8 which examined the prognostic significance of tumor morphotype, mostly reporting that PRCC had a better prognosis [80–90% 5-year cancer-specific survival (CSS)] as compared to other types of RCC with the exception of chromophobe RCC. However, some researchers have observed 5-year CSS as low as 50%9 and 61%3 for PRCC. Furthermore, a lack of independent association with outcome between the common histological types of RCC has been reported in multivariate analyses10,11. The issue is further complicated by the concept of two distinct morphological subtypes of PRCC, type 1 and type 2, which are claimed to be different in their clinical behavior and cytogenetic profile, with type 1 PRCC tending less often to present with locally advanced disease and with lower rates of metastases than type 2 PRCC12,13. However, several studies have challenged this subtyping scheme as an independent prognostic factor14,15,16. Despite being adopted by the World Health Organization (WHO) classification of renal tumors since 2004, this subtyping scheme remains controversial, with growing molecular evidence demonstrating that type 2 PRCC is genetically a heterogeneous group which includes other distinct entities17. Moreover, PRCC with mixed or overlapping features of type 1 and type 2 are frequently encountered18, suggesting that there is considerable variation in the morphologic spectrum of type 1 PRCC that has not been adequately addressed, adding to the difficulty in accurately classifying these tumors.
Here we conducted a single-institution study of nearly two hundred consecutive cases of PRCC with any prototypical type 1 components, with the longest clinical follow-up to date, to further refine the morphologic and molecular spectrum, identify features associated with aggressive behavior, and better define the long-term clinical course of true PRCC. We also investigated the molecular alterations in tumors with mixed type 1 and type 2 histologic features to explore the biological relationship between these two elements.
Materials and methods
Case selection
The WHO classification of renal tumors describes two morphological subtypes of PRCC, namely, type 1 and type 219,20. Per this description, type 1 tumors are composed of papillae lined by cells with low-grade nuclei, scant clear to amphophilic cytoplasm, arranged in a single layer while type 2 tumors contain pseudostratified cells with abundant eosinophilic cytoplasm and often, higher nuclear/nucleolar grade. After Institutional Review Board (IRB) approval, a clinical database search was undertaken to identify all nephrectomies performed at the Memorial Sloan Kettering Cancer Center (MSKCC) between 1995 and 2008 with a final diagnosis of PRCC. This time period was selected to ensure a large number of cases with prolonged clinical follow up. From all such cases where slides and blocks could be obtained, a thorough review was performed to identify cases with at least focal classic type 1 areas. Cases with type 2 features were included if any amount of clearly identifiable type 1 areas were present. Cases with histologic features raising suspicion for recently recognized entities with papillary architecture such as MiT family translocation RCC and FH-deficient RCC were not included in the study. This histologic review identified 201 patients with tumors fulfilling the morphologic inclusion criteria. For patients with multiple papillary RCCs resected at the same time, the largest tumor was used for histologic and survival analyses. Two patients with metastasis at initial presentation were excluded. Thus, a total of 199 patients were included in the study.
Clinicopathologic assessment
Clinical and follow-up information was obtained from prospectively maintained institutional databases or electronic medical records documenting age, gender, type of surgery (partial or radical nephrectomy), and vital status at follow-up. Gross pathology reports provided data regarding tumor size, laterality, focality, and location in the kidney. Pathological tumor stage (pT) was assigned according to the eighth edition of the American Joint Committee on Cancer (AJCC) cancer staging manual21. All cases that did not exhibit straightforward, well-defined morphological features were reviewed by three urological pathologists to reach a consensus. In each case, the following histopathologic features were evaluated by dedicated genitourinary pathologists.
Type 2 features
The presence or absence of type 2 features, as previously stated, were recorded. In tumors with mixed histology characterized by the presence of type 2 features, this was quantified as a percentage of tumor area. In addition, nuclear pseudostratification, stressed as a primary characteristic separating type 1 and type 2 tumors22 was independently noted and quantified.
Tumor grading
The WHO/ISUP grading system, which is mainly based on nucleolar grade, as well as the nuclear grade based on the Fuhrman grading system were used to evaluate tumor grade. Both the highest grade, as observed in at least two high-power microscopic fields, and the predominant grade, as the most prevalent (≥50%) grade, were documented.
Mitotic count
After review of each slide, the areas with maximum mitotic activity were chosen, and the mitotic count was documented as the number of mitotic figures per 10 high-power fields.
Lymphovascular invasion (LVI)
This was annotated as absent or present when the tumor involved vascular structures with the exclusion of the renal vein, its segmental branches, and the inferior vena cava.
Cytoarchitectural features
The architecture was captured as papillary, tubulo-papillary, sheet-like, or sarcomatoid and quantified when more than one type was present. In particular, sheet-like pattern was defined as areas of the tumor where discrete papillary or tubulopapillary architecture was replaced by contiguous growth of tumor cells that either represented tightly packed back-to-back papillary structures or a solid growth that lacked intervening vascular structures. Evaluated cytological features included the amount (scant vs moderate-abundant) and tinctorial qualities (clear vs eosinophilic-amphophilic) of the cytoplasm.
Other features
Multiple other parameters including the presence and amount of tumor circumscription, presence, extent, and invasion of tumor capsule, necrosis, siderophages, psammoma bodies, foamy macrophages, and fibrosis were also assessed. The presence of concurrent papillary adenoma(s) was recorded.
Molecular analysis
All archival slides from tumors with mixed type 1 and type 2 features were reviewed to select a subset of cases (n = 22) that had discrete areas of type 1 and type 2 morphology and sufficient tumor quantity for molecular analysis. DNA was extracted from the macro-dissected formalin-fixed paraffin-embedded (FFPE) tissue samples of paired type 1 and type 2 tumor areas as well as the matched normal kidney using QIAamp DNA FFPE Tissue Kit according to the manufacturer’s instructions. The total 66 DNA samples from these 22 cases were analyzed using our institutional sequencing platform MSK-IMPACT®, a version consisting of hybridization-capture of 468 cancer-associated genes and concordant tiling of heterozygous single nucleotide polymorphisms across the genome23 (Supplementary Table 1). Somatic mutations were called after private germline single-nucleotide variants (SNVs) detected in the paired normal sample were appropriately filtered out. The functional impacts of detected mutations were categorized as oncogenic/likely oncogenic and variants of unknown significance (VUS) using OncoKB (http://oncokb.org), a precision oncology knowledge base maintained at MSKCC24. Copy number analysis was conducted using FACETS (v0.5.6), an algorithm that allows detection of allele-specific copy-number events as well as deconvolution of the relative tumor/normal abundances in the sample (i.e., purity). Arm-level events were defined as copy-number changes occurring in a segment that comprised at least 50% of the chromosomal arm25. In order to estimate the fraction of the genome with copy-number alterations (CNAs), which is known to be a surrogate of genomic instability, we followed an approach similar to the one described by Endesfelder et al.26. Segments with a copy-number log ratio above or below 0.2 were considered to be altered. The length of the CNA segments was then compared against the total chromosomal length and this value was averaged across all the autosomal chromosomes (N = 22 autosomes).
Statistical analyses
To determine if any of the measured clinical and pathological characteristics were associated with CSS or progression-free survival (PFS), univariate Cox proportional hazards regression was employed. We visualized CSS and PFS for the entire cohort using Kaplan–Meier curves. Patient CSS was determined from the date of surgery until death from PRCC or death from other cause or until the most recent patient contact while PFS was determined from date of surgery until the date of recurrence or until the most recent patient contact. There were 4 patients who had a second surgery up to 11 months after the first surgery to remove a second and smaller PRCC tumor in the contralateral kidney. In these cases, we only analyzed data from the first nephrectomy and defined PFS and CSS from the date of their first nephrectomy. All tests were two-sided and a pre-rejection α < 0.05 was used to define statistical significance. Analyses were performed using Stata 15 (Stata Corp., College Station, TX) or R platform v3.5.0.
Results
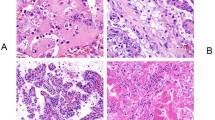
The pertinent clinicopathological characteristics are detailed in Table 1. Among the 199 patients, 133 (67%) underwent a partial nephrectomy. Multiple PRCC were detected in 28 patients, 14 of whom had bilateral disease. There were 95 patients (48%) whose tumors contained some type 2 morphology component, the amount of which ranged from 1% to 98%, with a median of 25% (IQR: 10%, 70%) (Fig. 1A–C). At least partial encapsulation was observed in 159 (80%) tumors, and 82 (41%) showed capsular invasion (Fig. 1D). While the predominant growth pattern was papillary and tubulopapillary as expected, sarcomatoid differentiation was seen in 4 (2%) tumors and sheet-like architecture was present in 11 (5.5%) cases (Fig. 1E, F). Microscopic necrosis was identified in 124 (63%) cases with a median of 4% (IQR 0, 15) tumor area. Mitotic counts of ≥ 1/10 high power fields (HPF) were seen in 48 (24%) of tumors, ranging from 1 to 24/10 HPF (median, 2/10HPF). Lymphovascular invasion was present in 6 (3%) patients. A majority of tumors showed a predominant WHO/ISUP grade of 1 or 2 [grade 1, n = 120 (60%), grade 2, n = 61 (31%)] and the remaining 18 (9%) were predominantly grade 3. Meanwhile, the distribution of tumors with any amount of highest nucleolar grade was as follows: grade 1, 34 (17%), grade 2, 81 (41%), grade 3, 80 (40%), and grade 4, 4 (2%).
A Classic type 1 histology. B Intermixed type 1 and type 2 areas associated with hemorrhage and necrosis, some pseudostratified eosinophilic cells in type 2 areas show marked hemosiderin deposition. C Intermixed low-grade type 1 area transitioning into type 2 area with higher nuclear grade. D Capsular invasion. E Sheet-like growth pattern. F Sarcomatoid differentiation.
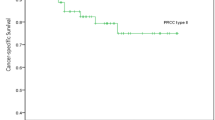
Ten patients developed metastasis and died of disease and 14 recurred. The median follow-up time among those who did not die of disease was 12.0 years (IQR: 8.6, 14.3). Figure 2A, B displays the overall Kaplan–Meier curves for progression-free and cancer-specific outcomes, respectively. The estimated probability of recurrence at 15 years of follow-up was 8% (95% CI 5%, 13%) and for cancer-specific death was 6% (95% CI 3%, 10%).
Table 2 summarizes the results of the univariate Cox regression analysis. We found no evidence that either presence of type 2 morphology or the percentage of type 2 morphology was associated with PFS or CSS rates (p = 0.2 and 0.4 for presence, p = 0.084 and 0.15 for percentage). On the other hand, tumor size >7 cm, pathologic stage pT3, LVI, and predominant WHO/ISUP grade 3 were all significantly associated with PFS and CSS. Additionally, measured as continuous variables, mitotic rate (≥1/10HPF), and percentages of sarcomatoid differentiation, sheet-like architecture, and circumscription were also found to be significantly associated with PFS and CSS (Table 2). All 6 patients who had LVI died of disease (HR = 114; 95% CI 28, 472; p < 0.0001). Interestingly, the highest WHO/ISUP grade did not show a significant association with either PFS or CSS (p = 0.3 and 0.7, respectively). Similarly, tests for association between the highest Fuhrman nuclear grade and either outcome did not meet conventional levels of significance (p = 0.10 and p = 0.069, respectively). Multivariate analysis could not be performed in our cohort due to the low rates of adverse events.
Among the cases with mixed type 1 and type 2 morphology, we identified a subset of 22 cases with distinct type 1 and type 2 areas and performed macro-dissection and paired comparison of molecular alterations (Supplementary Table 2). In total, 78 somatic mutations of 54 genes were identified in these 44 samples, including 12 genes that were recurrently mutated in at least 2 patients (Fig. 3A). TERT promoter mutation was the most common mutation, detected in 9 samples from 5 (23%) cases. Aside from MET activating mutations (2/22, 9%), a known driver mutation in PRCC, other recurrently mutated genes ARID1A, KDM6A, KMT2D, NFE2L2, APC, TP53, and LATS1, were implicated in histone methylation, SWI/SNF complex, response to oxidative stress, and other cancer-related pathways. Importantly, 61 of 78 (78%) detected mutations were shared between the paired type 1 and type 2 areas. The remaining 17 mutations were private, with 8 in type 1 areas and 9 in type 2 areas (Fig. 3B).
A Somatic mutations identified in the paired type 1 and type 2 areas. Mutated genes are listed on the left and denoted by individual rows. Paired samples from type 1 and type 2 areas of individual tumors are presented as columns and labeled at the bottom (T1-type 1; T2-type 2). Number of mutations detected in each sample (top) and number of cases with non-silent mutations detected on individual genes (right side) are listed. B Venn diagram depicting the numbers of shared and private mutations detected. C Frequency of copy number changes (gain-red; loss-blue) across chromosomes 1-22 in type 1 and type 2 areas.
The copy number alterations observed in both type 1 and type 2 areas were comparable to those of The Cancer Genome Atlas (TCGA) results in type 1 PRCC17,27. Gains of chromosomes 7 and 17 were the most frequent arm-level alterations, followed by gains of chromosomes 12, 16, and 20. A significant subset (~40%) of cases also harbored chromosome 3 gain. Overall, the copy number alterations observed in type 1 and type 2 areas were very similar (Fig. 3C), and there was no significant difference between the fractions of copy number altered chromosomal regions detected between type 1 and type 2 areas (Supplementary Fig 1).
At the individual case level, in all except one case in which no somatic mutations were detected, 1 to 7 shared mutations were found between distinct regions of type 1 and type 2 (Fig. 4). Despite the morphologic difference between type 1 and type 2 areas, identical somatic mutations were detected in 11 (50%) cases and differed only by one mutation in another 6 (27%) (Fig. 3A). Interestingly, two pathogenic TP53 mutations were found in the analysis as a private mutation only in type 2 areas (Fig. 4D). One case harbored KRAS G12V activating mutation in both type 1 and type 2 areas (Fig. 5).
Discussion
Renal tumors with papillary architecture were first documented by Pierre Rayer in 184228. However, it was not until 1976 that Mancilla-Jiminez et al.1 described PRCC as a group of tumors with distinct morphology and clinical behavior. More than a decade later, at a consensus meeting held at Heidelberg, Germany, involving urologists, pathologists, and geneticists, based on evidence collected from several studies published in the intervening years4,12,29,30,31,32, PRCC was formally adopted as a subtype of renal carcinoma in a classification scheme that included clear cell, chromophobe, collecting duct and unclassified renal cell carcinomas33. Later that year, another workgroup meeting took place at Rochester, USA, under the auspices of the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC)34 adapted the classification guidelines proposed at the Heidelberg conference. The proposals put forth by the Heidelberg and Rochester groups on the classification of renal cell tumors, was the basis of the 2004 WHO classification of renal tumors19. PRCC has since then been established as the second most common subtype of renal carcinoma and several studies have shown its clinical behavior to be less aggressive than clear cell RCC but worse than chromophobe carcinoma2,3,5,6,8,10,11,34,35,36.
It was also increasingly recognized that tumors with papillary architecture exhibited heterogeneity in both morphological features and clinical behavior. As described in the 2004 WHO classification of renal tumors35, the classic features of PRCC consist of papillary cores lined by a single layer of small cuboidal cells with small oval bland nuclei, inconspicuous nucleoli, scant pale cytoplasm, and a lack of mitotic activity. Additional features include a tubulopapillary architecture, foamy macrophages in the papillary cores, edema and psammoma bodies as well as frequent hemorrhage, necrosis, and cystic degeneration. However, nuclear pseudostratification, moderate to abundant eosinophilic cytoplasm, and higher nuclear grade with prominent nucleoli were identified in some papillary tumors that in general also lacked edema, foamy macrophages, and psammoma bodies. While earlier studies1,4,29 included these features as part of the spectrum of papillary carcinomas, Delahunt et al.12 were the first to categorize PRCC into type 1 and type 2, with the former referring to tumors with classic features and the latter to the eosinophilic, pseudostratified entities. In a report of 105 papillary carcinomas, they found significant differences between the two types by univariate analysis with type 2 tumors exhibiting larger size, higher pathologic stage, and higher nuclear grade. In a subsequent study by the same authors13, a comparative survival analysis of 50 type 1 and 16 type 2 tumors with a 5-year follow-up period was performed. Multivariate analysis showed tumor type and stage were significant predictors of survival while nuclear grade was not. However, details on the categorization of tumors with mixed morphology as well as cut-off points for typing such tumors were not specified. Following these formative works, several authors investigated the prognostic significance of subtyping PRCC. However, no definite conclusion could be drawn from several multivariate survival analyses14,15,16,37,38. Allory et al.37 and Pignot et al.38 in agreement with Delahunt et al.12,13 demonstrated the significance of tumor type as a predictor of overall survival while concluding nuclear grade was not. In contrast, the work of other researchers including Mejean et al.14, Gonterro et al.9, Klatte et al.15, Antonelli et al.39 and Sukov et al.16, could not validate these findings in studies that had greater case numbers and follow up intervals. On the other hand, the percentages of type 1 vs. type 2 tumors in these reported cohorts exhibited a broad range (type 1 PRCC 31–76%), suggesting interobserver viability and differences in criteria used for subtyping. Meanwhile, it also remained unclear how to apply subtyping criteria in PRCC with mixed type 1 and type 2 histology, which represented a significant portion of PRCC18.
At the molecular level, early studies by Kovacs et al.29,31,40 showed PRCC is characterized by gains of chromosome 7 and 17, loss of Y chromosome (in men), as well as frequent additional trisomies of 12, 16, and 20. Subsequent studies largely confirmed these findings, but also revealed variable results when comparing cytogenetic aberrations between type 1 and type 2 tumors, with type 2 tumors often showing less prevalence of trisomy 7/17 and more complex cytogenetic abnormalities39,40,41,42,43,44,45. While it is known that hereditary PRCC, a rare predisposing disorder associated with activating germline mutations of MET gene, exhibits type 1 features46, somatic MET mutations occur only in 13–15% of sporadic cases47,48. Importantly, of increasing relevance in recent years is the discovery of RCCs with prominent papillary architecture and type 2 features but harboring distinct genetic alterations that warrant their unique categorization in the current classification scheme. Studies of PRCC in the past certainly included these entities as type 2 PRCC, which possibly contributed to data set contamination and skewed prognostic data. These recently recognized entities include FH-deficient RCC or hereditary leiomyomatosis and renal cell carcinoma syndrome (HLRCC)-associated RCC when characterized by germline FH aberrations, MiTF altered RCCs, ALK translocation associated RCC, tuberous sclerosis complex (TSC) associated RCC, and other rare types of RCC with papillary architecture. The recent comprehensive molecular characterization of PRCC by TCGA further demonstrated the molecular heterogeneity in type 2 tumors while revealing a nearly universal gain of chromosomes 7 and 17 and less frequent gain of chromosomes 2, 3, 12, 16, and 20 in morphologically defined type 1 cases17. Among the type 2 and unclassified (type unspecified) tumors examined in the TCGA PRCC study, aside from cases harboring specific molecular alterations (e.g., FH mutation, TFE3/TFEB fusion, etc.) that indicate distinct molecular subtypes, it is interesting to note that some remaining tumors shared copy number and/or other molecular features found in type 1 PRCC, while others not. The molecular evidence of heterogeneity in morphologically defined type 2 PRCC strongly supports a need to reassess the clinical value of subtyping in the context of contemporary classification systems and to better delineate the morphologic spectrum of PRCC.
To evaluate the long-term clinical course of true PRCC in light of the prevailing uncertainties of type 2 morphology, we selected cases with clearly identifiable type 1 features as described in the methods section. This inclusion criterion was based on the premise that the presence of prototypical morphology, even if focal, defines tumor classification, an approach commonly applied in the diagnosis of clear cell RCC and classic chromophobe RCC. For PRCC, we hypothesized that classic type 1 histology is the low grade and prototypical morphology of a broader morphologic spectrum of PRCC that can include type 2 features. This study design allowed us to assess the prognostic values of type 2 features as compared to other clinicopathologic parameters in a large cohort of PRCC with long follow-up data and likely similar pathogenesis. To test this hypothesis, we also aimed to understand the biological relationship between these two types of morphologies in cases with mixed features.
Our analysis of 199 consecutive cases with at least focal classical PRCC type 1 features showed very low (8% and 6% respectively) estimated probabilities for recurrence and cancer-specific death at 15 years of follow-up. When defined as such, our finding suggests PRCC has a prognosis comparable to the reported 5-year survival rates of chromophobe RCC (80% – 100%)49. Furthermore, we observed a lack of significant association between the presence or amount of type 2 features and survival parameters even with a median follow-up of 12 years. We found nearly half the cases in the present study demonstrating mixed type 1 and type 2 features in varying proportions. In a majority of these cases, as well as in other cases containing areas that did not meet the complete definitional criteria of type 2, a spectrum of morphological features could be identified, including type 1 areas that exhibited varying amounts of eosinophilic cytoplasm, unclassifiable areas containing high-grade monolayered nuclei or low-grade pseudostratified cells with abundant eosinophilic cytoplasm and several such combinations. Furthermore, high-grade nuclei were also observed in several tumors that would otherwise be considered type 1 based on architecture, cytoplasm, stratification, and stromal features. Delahunt and colleagues12,13 who originally proposed subtyping, have also stressed in a more recent publication49 that the classification of PRCC as type 1 or type 2 should primarily be based on the presence or absence of pseudostratification of tumor nuclei due to morphological overlap between the two types. However, we did not find sufficient evidence to suggest that the percentage of pseudostratified nuclei was associated with PFS or CSS (p = 0.8 and 1 respectively).
To investigate the biological relationship between morphologically distinct type 1 and type 2 regions in a given tumor, we subjected spatially separate type 1 and type 2 regions from 22 cases with mixed morphology to a targeted NGS platform and interrogated alterations in 468 cancer-related genes, including a vast majority of those implicated in the pathogenesis of RCC. The recurrently mutated genes we detected in this subset of cases are in line with the TCGA results, except for the TERT promoter mutations, an alteration that was not analyzed in the TCGA KIRP study17. The mutation analysis demonstrated largely shared somatic mutations between distinct regions of type 1 and type 2 features, with 78% of detected mutations shared between the paired regions. Interestingly, not only are these paired regions clonally related (all shared some mutations), the frequencies of most recurrent mutations were also similar in type 1 and type 2 areas, suggesting that type 2 areas may represent a morphologic spectrum rather than a later stage of the tumor progression, at least in some cases. However, both TP53 pathogenic mutations found in this analysis were a private mutation of the type 2 area, consistent with it being a later molecular event of clonal evolution that could theoretically drive tumor progression and lead to decreased survival in PRCC27. Several recent studies of clear cell RCC suggested TERT promoter mutations may define a small subset of tumors with aggressive behavior50,51. Although 3 of these 5 patients harboring TERT promoter mutations in our cohort died of disease during the follow-up period, additional studies are required to clarify the clinical significance of this alteration. One case in our cohort harbored a shared KRAS activating mutation in the paired type 1 and 2 areas, concurrent with a shared NFE2L2 (also known as NRF2) mutation and a private ARID1A mutation (type 2 area). This case did not exhibit the morphology of papillary renal neoplasm with reverse polarity, a group of tumors frequently harbor KRAS mutations and also may have been referred to in the literature as “oncocytic low-grade papillary RCC” and “type 4 PRCC”52,53,54,55,56.
The copy number alterations observed in this subset of PRCC were also similar to those of TCGA results in type 1 PRCC, with gains of chromosomes 7 and 17 being most common, followed by less frequent gains of chromosomes 12, 16, 20, and 3. Furthermore, most of these copy number alterations were shared by the distinct type 1 and type 2 pairs in a given case. Taken together, although the molecular analysis we conducted was limited to a targeted panel of 468 commonly mutated cancer-related genes, our results support that PRCC with mixed type 1 and type 2 features, despite the presence of type 2 morphology in varying proportions, is molecularly consistent with a single tumor type with either morphologic variability or clonal evolution. This finding is in line with some previous studies that demonstrated cytogenetic/molecular overlap or clonal evolution between the type 1 and type 2 morphology44,45,57.
We also evaluated in detail the prognostic value of a range of clinicopathologic parameters in this cohort. Tumor grading in PRCC has been a controversial issue until the more recent adoption of the 4-tiered WHO/ISUP grading system58. As the cases in our study were graded at the time of clinical diagnosis using the criteria proposed by Fuhrman et al.59, we included both Fuhrman and WHO/ISUP grading system to assess their performance in this cohort with long follow-up. We also compared the highest vs. the predominant grade in the analysis. The current study showed that a predominantly high grade (grade 3) in both WHO/ISUP and Fuhrman grading systems is significantly associated with PFS and CSS [(p = 0.010, 0.004) and (p = 0.013, 0.030) respectively] while less-than-predominant high grade nucleolar and nuclear features were not. The latter non-predominant high-grade features were often associated with cytoplasmic eosinophilia, with hemorrhage, degenerative type necrosis and abundant cytoplasmic hemosiderin deposition. We speculate whether these changes may induce nuclear and nucleolar changes as a reactive phenomenon. It is also noteworthy that in several cases, the transition from type 1 to type 2 areas appeared to be closely related to zones of hemorrhage and degenerative changes (Fig. 1B). We did not find a significant prognostic impact associated with the presence or amount of hemosiderin-laden tumor cells.
It should be noted that despite the overall favorable prognosis, some PRCC with type 1 morphology, behave aggressively, as evidenced by the ten cases of tumor-related mortality in our cohort. Although we did not perform multivariable analysis owing to the low event rate, several parameters, in addition to tumor size and predominant nuclear and nucleolar grade, were significantly associated with PFS and CSS by univariate analysis. In agreement with other studies that showed association of higher tumor stage with adverse clinical outcome9,13,14,16,37,38,60,61, we found pathologic stage 2 and 3 had cancer-specific mortality rates significantly greater than pT1 (p = 0.034 and 0.0003 respectively). Microvascular invasion has been shown to predict PRCC survival by few authors14,38,60. We observed a significant association between microvascular invasion and CSS (p = <0.0001). Of note, all 6 patients with microvascular invasion in our cohort died of disease. The presence of tumor capsular invasion had a higher risk of progression (p = 0.044) and lack of tumor circumscription showed a significant association with PFS and CSS (p = 0.03 and p = 0.0002 respectively). In line with two recent studies that showed solid architecture or sheet-like growth as a significant prognostic indicator of PRCC61,62, we found the presence of sheet-like growth pattern was also associated with both outcome measures (p = 0.015 for PFS and p = 0.005 for CSS). Aside from the solid/sheet-like growth pattern, hobnail, micropapillary, and microcystic features have also been identified to be of prognostic significance and often co-occur61,62. These histologic features represent important histologic patterns to be further validated for their independent prognostic values. Moreover, increased mitotic activity is not a prominent feature of PRCC, with only isolated reports demonstrating prognostic relevance of tumor proliferation as measured by Ki67 activity9,13. However, when present (≥1/10HPF), mitotic rate was significantly associated with survival (p = <0.0001). In our study, the average mitotic rate was 5.45 /10 HPF among those who recurred versus 0.43 /10 HPF in those who did not recur. Another morphological feature in PRCC that is uncommonly described and worth mentioning is the presence of low-grade spindling of the tumor cells. Low grade spindling is seen in some cases of type 1 PRCC63, and in our series did not demonstrate adverse prognostic significance (data not shown). It is important not to associate this finding with true sarcomatoid change. Unlike the latter, low-grade spindling demonstrates papillary growth pattern with minimal nuclear atypia. True sarcomatoid change was, as expected, significantly associated with adverse clinical outcome (p = <0.0001). Although tumor necrosis has been shown to have prognostic significance in clear cell RCC, most studies have concluded that it does not have the same predictive value in PRCC2,12,37. However, two recent reports have observed otherwise60,64. Some authors have reported lack of tumor macrophages37,38,65 and increase in stromal fibrosis37 to be associated with adverse clinical behavior in PRCC. Although we could not demonstrate significant association between fibrosis and tumor behavior, the lack of macrophages or foam cells appeared to be significantly associated with CSS in our cohort (p = 0.029). It is worth noting that given the long follow-up period of the study, we included 5 cases of PRCC that were smaller than 1.5 cm (range 1.0–1.4 cm), which would not have met the new size criterion defined by the 2016 WHO classification58. The inclusion of these small tumors did not change the results of the association analysis.
As we focused on investigating PRCC with at least focal type 1 morphologic features, one limitation of our study is that we did not examine cases with pure type 2 or other unusual features but exhibiting papillary architecture. Although many of these may indeed represent cases distinct from PRCC, some currently recognized variants of PRCC, such as papillary renal neoplasm with reverse polarity and Warthin-like PRCC56, were not included in the analysis. It is notable that this study contained almost half the tumors with variable type 2 features, some with the proportion approaching 100%. All the tumors, however, were true papillary RCC as defined by the 4th edition of the WHO classification.
In summary, we present a comprehensive clinicopathological and molecular analysis of a large series of PRCC diagnosed and treated at a single institution with long-term clinical follow-up. Although limited by low event rate, univariate analysis showed predominant high WHO/ISUP grade 3 is significantly associated with clinical outcome, but focal high-grade features are not. In addition, larger tumor size, higher pathologic stage, increased mitotic activity, microvascular invasion, sarcomatoid and sheet-like architectures appear to be significantly associated with outcome in type 1 PRCC. We conclude that PRCC with any component of type 1 morphology, regardless of the presence or amount of type 2 features (up to 98% in this study), have excellent long-term clinical outcomes. Molecular study of a subset of cases with mixed features revealed type 2 areas largely shared molecular alterations with type 1 areas, supporting the paradigm that type 2 histologic features likely represent either morphologic variability or clonal evolution in type 1 PRCC. It also supports the fundamental hypothesis of our study and reiterates the notion that PRCC with any classic type 1 regions is best classified as type 1 PRCC and assigned the appropriate WHO/ISUP nucleolar grade. Finally, it provides further evidence that tumors previously classified as type 2 PRCC are a heterogeneous group of neoplasms rather than an entity and that some represent high-grade variants of type 1 PRCC. It is understandable why the Genitourinary Pathology Society (GUPS)56 has recommended its removal from the classification of renal tumors, a recommendation that the World Health Organization is likely to follow in its forthcoming edition.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mancilla-Jimenez, R., Stanley, R. J. & Blath, R. A. Papillary renal cell carcinoma: a clinical, radiologic, and pathologic study of 34 cases. Cancer 38, 2469–2480 (1976).
Moch, H. et al. Prognostic utility of the recently recommended histologic classification and revised TNM staging system of renal cell carcinoma: a Swiss experience with 588 tumors. Cancer 89, 604–614 (2000).
Ljungberg, B., Alamdari, F. I., Stenling, R. & Roos, G. Prognostic significance of the Heidelberg classification of renal cell carcinoma. Eur. Urol. 36, 565–569 (1999).
Amin, M. B. et al. Papillary (chromophil) renal cell carcinoma: histomorphologic characteristics and evaluation of conventional pathologic prognostic parameters in 62 cases. Am. J. Surg. Pathol. 21, 621–635 (1997).
Cheville, J. C., Lohse, C. M., Zincke, H., Weaver, A. L. & Blute, M. L. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am. J. Surg. Pathol. 27, 612–624 (2003).
Amin, M. B. et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am. J. Surg. Pathol. 26, 281–291 (2002).
Beck, S. D. et al. Effect of papillary and chromophobe cell type on disease-free survival after nephrectomy for renal cell carcinoma. Ann. Surg. Oncol. 11, 71–77 (2004).
Leibovich, B. C. et al. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J. Urol. 183, 1309–1316 (2010).
Gontero, P. et al. Prognostic factors in a prospective series of papillary renal cell carcinoma. BJU Int. 102, 697–702 (2008).
Patard, J. J. et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J. Clin. Oncol. 23, 2763–2771 (2005).
Capitanio, U. et al. A critical assessment of the prognostic value of clear cell, papillary and chromophobe histological subtypes in renal cell carcinoma: a population-based study. BJU Int. 103, 1496–1500 (2009).
Delahunt, B. & Eble, J. N. Papillary renal cell carcinoma: a clinicopathologic and immunohistochemical study of 105 tumors. Mod. Pathol. 10, 537–544 (1997).
Delahunt, B. et al. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum. Pathol. 32, 590–595 (2001).
Mejean, A. et al. Prognostic factors for the survival of patients with papillary renal cell carcinoma: meaning of histological typing and multifocality. J. Urol. 170, 764–767 (2003).
Klatte, T. et al. Cytogenetic and molecular tumor profiling for type 1 and type 2 papillary renal cell carcinoma. Clin. Cancer Res. 15, 1162–1169 (2009).
Sukov, W. R., Lohse, C. M., Leibovich, B. C., Thompson, R. H. & Cheville, J. C. Clinical and pathological features associated with prognosis in patients with papillary renal cell carcinoma. J. Urol. 187, 54–59 (2012).
Linehan, W. M. et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N. Engl. J. Med. 374, 135–145 (2016).
Chevarie-Davis, M. et al. The morphologic and immunohistochemical spectrum of papillary renal cell carcinoma: study including 132 cases with pure type 1 and type 2 morphology as well as tumors with overlapping features. Am. J. Surg. Pathol. 38, 887–894 (2014).
Delahunt B., Eble J. N. Papillary renal cell carcinoma. In: Eble J. N., Sauter G., Epstein J. I., Sesterhenn I. A., (eds). World Health Organization classification of tumours pathology and genetics of the tumours of the urinary system and male genital organs. (IARC Press, Lyon, 2004).
Delahunt B., et al. Papillary renal cell carcinoma. In: Moch H., Humphrey P. A., Ulbright T. M., Reuter V., (eds). WHO classification of tumours of the urinary system and male genital organs. (IARC, Lyon, 2016).
Amin M. B., et al., (eds). AJCC cancer staging manual, (Springer International Publishing, 2017).
Delahunt, B. et al. The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am. J. Surg. Pathol. 37, 1490–1504 (2013).
Cheng, D. T. et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): a hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J. Mol. Diagn. 17, 251–264 (2015).
Chakravarty, D. et al. OncoKB: a precision oncology knowledge base. JCO Precis. Oncol. 2017, 1–16 (2017).
Shen, R. & Seshan, V. E. FACETS: allele-specific copy number and clonal heterogeneity analysis tool for high-throughput DNA sequencing. Nucleic Acids Res. 44, e131 (2016).
Endesfelder, D. et al. Chromosomal instability selects gene copy-number variants encoding core regulators of proliferation in ER+ breast cancer. Cancer Res. 74, 4853–4863 (2014).
Ricketts, C. J. et al. The cancer genome atlas comprehensive molecular characterization of renal cell carcinoma. Cell Rep. 23, 313–326.e315 (2018).
Delahunt, B. & Eble, J. N. History of the development of the classification of renal cell neoplasia. Clin. Lab. Med. 25, 231–246 (2005).
Kovacs, G. Papillary renal cell carcinoma. A morphologic and cytogenetic study of 11 cases. Am. J. Pathol. 134, 27–34 (1989).
Mydlo, J. H. & Bard, R. H. Analysis of papillary renal adenocarcinoma. Urology 30, 529–534 (1987).
Kovacs, G., Wilkens, L., Papp, T. & de Riese, W. Differentiation between papillary and nonpapillary renal cell carcinomas by DNA analysis. J. Natl. Cancer Inst. 81, 527–530 (1989).
Corless, C. L. et al. Papillary renal cell carcinoma: quantitation of chromosomes 7 and 17 by FISH, analysis of chromosome 3p for LOH, and DNA ploidy. Diagn. Mol. Pathol. 5, 53–64 (1996).
Kovacs, G. et al. The Heidelberg classification of renal cell tumours. J. Pathol. 183, 131–133 (1997).
Storkel, S. et al. Classification of renal cell carcinoma: Workgroup No. 1. Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC). Cancer 80, 987–989 (1997).
Eble J. N., Sauter G., Epstein J. I., Sesterhenn I. A., (eds). World Health Organization classification of tumours. Pathology and genetics of tumours of the urinary system and male genital organs. (IARC Press, Lyon, 2004).
Srigley, J. R. et al. The International Society of Urological Pathology (ISUP) vancouver classification of renal neoplasia. Am. J. Surg. Pathol. 37, 1469–1489 (2013).
Allory, Y., Ouazana, D., Boucher, E., Thiounn, N. & Vieillefond, A. Papillary renal cell carcinoma. Prognostic value of morphological subtypes in a clinicopathologic study of 43 cases. Virchows Arch. 442, 336–342 (2003).
Pignot, G. et al. Survival analysis of 130 patients with papillary renal cell carcinoma: prognostic utility of type 1 and type 2 subclassification. Urology 69, 230–235 (2007).
Antonelli, A. et al. Cytogenetic features, clinical significance and prognostic impact of type 1 and type 2 papillary renal cell carcinoma. Cancer Genet Cytogenet 199, 128–133 (2010).
Kovacs, G., Fuzesi, L., Emanual, A. & Kung, H. F. Cytogenetics of papillary renal cell tumors. Genes Chromosomes Cancer 3, 249–255 (1991).
Kattar, M. M. et al. Clinicopathologic and interphase cytogenetic analysis of papillary (chromophilic) renal cell carcinoma. Mod. Pathol. 10, 1143–1150 (1997).
Jiang, F. et al. Chromosomal imbalances in papillary renal cell carcinoma: genetic differences between histological subtypes. Am. J. Pathol. 153, 1467–1473 (1998).
Brunelli, M., Eble, J. N., Zhang, S., Martignoni, G. & Cheng, L. Gains of chromosomes 7, 17, 12, 16, and 20 and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod. Pathol. 16, 1053–1059 (2003).
Gunawan, B. et al. Cytogenetic and morphologic typing of 58 papillary renal cell carcinomas: evidence for a cytogenetic evolution of type 2 from type 1 tumors. Cancer Res. 63, 6200–6205 (2003).
Balint, I., Szponar, A., Jauch, A. & Kovacs, G. Trisomy 7 and 17 mark papillary renal cell tumours irrespectively of variation of the phenotype. J. Clin. Pathol. 62, 892–895 (2009).
Zbar, B. et al. Hereditary papillary renal cell carcinoma. J. Urol. 151, 561–566 (1994).
Schmidt, L. et al. Novel mutations of the MET proto-oncogene in papillary renal carcinomas. Oncogene 18, 2343–2350 (1999).
Durinck, S. et al. Spectrum of diverse genomic alterations define non-clear cell renal carcinoma subtypes. Nat. Genet. 47, 13–21 (2015).
Delahunt B, Grignon DJ, Eble JN. Tumors of the kidney. In: Amin MB, Grignon DJ, Srigley JR, Eble JN, editors. Urological Pathology.
Hosen, I. et al. TERT promoter mutations in clear cell renal cell carcinoma. Int. J. Cancer 136, 2448–2452 (2015).
Casuscelli, J. et al. Characterization and impact of TERT promoter region mutations on clinical outcome in renal cell carcinoma. Eur. Urol. Focus 5, 642–649 (2019).
Al-Obaidy, K. I. et al. Papillary renal neoplasm with reverse polarity: a morphologic, immunohistochemical, and molecular study. Am. J. Surg. Pathol. 43, 1099–1111 (2019).
Al-Obaidy, K. I. et al. Recurrent KRAS mutations in papillary renal neoplasm with reverse polarity. Mod. Pathol. 33, 1157–1164 (2020).
Kunju, L. P., Wojno, K., Wolf, J. S. Jr, Cheng, L. & Shah, R. B. Papillary renal cell carcinoma with oncocytic cells and nonoverlapping low grade nuclei: expanding the morphologic spectrum with emphasis on clinicopathologic, immunohistochemical and molecular features. Hum. Pathol. 39, 96–101 (2008).
Saleeb, R. M. et al. Toward biological subtyping of papillary renal cell carcinoma with clinical implications through histologic, immunohistochemical, and molecular analysis. Am. J. Surg. Pathol. 41, 1618–1629 (2017).
Trpkov, K. et al. New developments in existing WHO entities and evolving molecular concepts: the Genitourinary Pathology Society (GUPS) update on renal neoplasia. Mod. Pathol. 34, 1392–1424 (2021).
Yang, X. J. et al. A molecular classification of papillary renal cell carcinoma. Cancer Res. 65, 5628–5637 (2005).
Moch H., Humphrey P., Ulbright T., Reuter V., (eds). WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4 edn. (International Agency for Research on Cancer (IARC), France, 2016).
Fuhrman, S. A., Lasky, L. C. & Limas, C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am. J. Surg. Pathol. 6, 655–663 (1982).
Klatte, T. et al. Development and external validation of a nomogram predicting disease specific survival after nephrectomy for papillary renal cell carcinoma. J. Urol. 184, 53–58 (2010).
Yang, C., Shuch, B., Kluger, H., Humphrey, P. A. & Adeniran, A. J. High WHO/ISUP grade and unfavorable architecture, rather than typing of papillary renal cell carcinoma, may be associated with worse prognosis. Am. J. Surg. Pathol. 44, 582–593 (2020).
Chan E., et al. Papillary renal cell carcinoma with microcystic architecture is strongly associapted with extrarenal invasion and metastatic disease. Am. J. Surg. Pathol. https://doi.org/10.1097/PAS.0000000000001802 (2021).
Argani, P., Netto, G. J. & Parwani, A. V. Papillary renal cell carcinoma with low-grade spindle cell foci - A mimic of mucinous tubular and spindle cell carcinoma. Am. J. Surg. Pathol. 32, 1353–1359 (2008).
Pichler, M. et al. Histologic tumor necrosis is an independent prognostic indicator for clear cell and papillary renal cell carcinoma. Am. J. Clin. Pathol. 137, 283–289 (2012).
Hutterer, G. C. et al. Tumour-associated macrophages might represent a favourable prognostic indicator in patients with papillary renal cell carcinoma. Histopathology 63, 309–315 (2013).
Acknowledgements
We gratefully acknowledge the members of the Molecular Diagnostics Service in the Department of Pathology, the Integrated Genomics Operation and Bioinformatics Core, and the Marie-Josée and Henry R. Kravis Center for Molecular Oncology of MSKCC. The study is supported by Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748).
Author information
Authors and Affiliations
Contributions
P.M., L.J., Y.B.C., S.K.T., and V.E.R. designed the study. P.M., L.J., N.B., H.A.A., S.W.F., A.G., J.S., S.J.S., A.A.H, P.R., Y.B.C., S.K.T., and V.E.R. identified and provided patient data. P.M., L.J., R.G.D., M.A., Y.B.C., S.K.T., and V.E.R. provided acquisition, analysis, and interpretation of data. P.M., L.J., and Y.B.C. drafted the manuscript; all authors critically read, edited, and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the MSKCC Institutional Review Board. This study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Murugan, P., Jia, L., Dinatale, R.G. et al. Papillary renal cell carcinoma: a single institutional study of 199 cases addressing classification, clinicopathologic and molecular features, and treatment outcome. Mod Pathol 35, 825–835 (2022). https://doi.org/10.1038/s41379-021-00990-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00990-9
This article is cited by
-
Papillary Renal Cell Carcinoma: A Review of Prospective Clinical Trials
Current Treatment Options in Oncology (2023)
-
A novel molecular signature identifies mixed subtypes in renal cell carcinoma with poor prognosis and independent response to immunotherapy
Genome Medicine (2022)