Abstract
A subset of clinically benign uterine polyps shows atypical morphologic features worrisome for, but not diagnostic of, Mullerian adenosarcoma. We report clinicopathologic data for 63 polyps from 58 women with atypical morphologic features including abnormal architecture, abnormal periglandular stroma, stromal atypia, and mitoses >2 per 10 hpf. Four (11%) of 36 women with follow-up tissue sampling had residual/recurrent atypical polyp. Twelve (27%) of 44 women underwent hysterectomy subsequent to a diagnosis of atypical polyp. No patient developed adenosarcoma over median follow-up of 150 months. Twenty-one primary atypical polyps underwent molecular profiling. Five (24%) harbored chr 12q13-15 gain or amplification, 9/20 (45%) harbored chr 6q25.1 gain, and 7/21 (33%) had no significant copy number alterations. Gains of chr 1q, chr 8p12, and chr 10q11.21-23, amplifications of chr 12q24.12-13, chr 15p24.1-26.1, and chr 18q21.33, and loss of chr 7 and chr 11q21 were each seen in a single polyp. Mean tumor mutational burden was 3.1 (range, 0.76–8.365) mutations/Mb. Pathogenic point mutations were identified in 12/20 (60%) primary atypical polyps. We propose the term “atypical uterine polyps” for these lesions, which show biologic overlap with early Mullerian adenosarcoma but lack molecular alterations characteristic of clinically aggressive adenosarcoma and appear to follow a benign clinical course. Conservative management with close clinical follow-up and repeat sampling can be considered for these lesions, when clinically appropriate.
Similar content being viewed by others
Introduction
Uterine polyps are benign monoclonal mesenchymal neoplasms1, comprising fibrous stroma admixed with histologically unremarkable non-neoplastic endometrial or endocervical glands. They occur across a wide age range but are most often seen in the perimenopausal period2,3,4. Multiple synchronous or metachronous polyps are not uncommon and may, in some cases, represent a single, clonal, clinically benign neoplasm5.
Endometrial polyps fall into distinct molecular subgroups6. Approximately half harbor recurrent rearrangements of chr 6p21 or chr 12q13-15, resulting in overexpression of mesenchymal growth factors HMGA1 and HMGA2, respectively1,6,7,8,9,10,11,12,13. Amplification of chr 12q13-15 has been described in a single endometrial polyp14 but is considered exceptionally rare. Conversely, approximately half of endometrial polyps show no cytogenetic abnormalities6 and may be related to altered estrogen signaling or to recurrent pathogenic point mutations in oncogenes, including KRAS, PPP1R2A, and ARHGAP3515,16. However, the precise pathogenic role of these point mutations is unclear15,17. The pathogenesis of endocervical polyps is less well characterized, but may also be related to excess estrogen3.
Uterine adenosarcoma is a malignant neoplasm of endometrial or, less often, endocervical stroma18,19. Like conventional benign uterine polyps, adenosarcoma presents as a polypoid mass and occurs across a wide age range, most frequently in the perimenopausal or early postmenopausal period18,19,20,21,22. However, adenosarcomas are, on average, larger than benign uterine polyps18,19,23 and show malignant histologic features, including phyllodiform architecture, periglandular cuffing by hypercellular stroma with subepithelial stromal condensation, stromal atypia, and increased stromal mitoses18,19. Most uterine adenosarcomas show relatively indolent behavior, with an overall 20–30% risk of recurrence18,22,24. However, a subset show features associated with more aggressive clinical behavior, including myometrial invasion, sarcomatous overgrowth, or high-grade stromal atypia18,19,22,25,26.
Uterine adenosarcomas are molecularly heterogeneous. Recurrent copy number alterations have been reported, including amplification of chr 12q13-15 (including MDM2, CDK4, and HMGA2), TERT, MYBL1, and BCL223,27,28,29,30. However, each of these alterations is seen in a minority of adenosarcomas. Recurrent point mutations in BCOR, ROS1, TP53, and ATRX are also described in minor subsets23,27,30, and TP53 and ATRX mutations are associated with high-grade morphology and adverse outcome23,26.
The vast majority of histologically banal endometrial and endocervical polyps follow a benign clinical course. However, recurrent endocervical20,31,32 or endometrial18 polyps may precede diagnosis of adenosarcoma, suggesting that uterine adenosarcoma may in some cases develop from a histologically unremarkable polyp. A potential relationship between banal polyps and adenosarcoma is further suggested by a subset of uterine polyps with intermediate histologic features, which are worrisome for adenosarcoma, but quantitatively or qualitatively insufficient for an adenosarcoma diagnosis. Such worrisome histologic features can include abnormal architecture, abnormal periglandular stroma, stromal atypia, and/or increased mitotic activity, which may be focal or diffuse within the polyp. These atypical uterine polyps pose a considerable diagnostic problem with significant therapeutic implications, particularly in women of childbearing age. In an earlier study of 27 women with atypical uterine polyps, 2 had a recurrent atypical polyp on follow-up, but none progressed to adenosarcoma or had an adverse disease-related outcome33. However, clinicopathologic data on atypical uterine polyps is confined to a single study, and their molecular features have not been described.
We hypothesized that atypical uterine polyps could represent (1) incipient adenosarcomas, (2) unusual morphologic variants of conventional uterine polyps, (3) a distinct molecular entity, or (4) a heterogeneous group comprising a combination of these. To examine these hypotheses, we evaluated 22 atypical uterine polyps by targeted next-generation sequencing (NGS), fluorescence in situ hybridization (FISH), or both. To better characterize their behavior, we also provide clinicopathologic data on 58 women with atypical uterine polyps.
Materials and methods
Cohort
This study was approved by the institutional review board at Brigham and Women’s Hospital. The cohort comprised 63 (58 primary; 5 residual/recurrent) atypical uterine polyps from 58 women (including additional follow-up information on 29 previously reported atypical uterine polyps from 27 women33). Novel cases were identified from the electronic pathology database using combinations of the terms (1) “polyp”; (2) “atypical” or “unusual”; and (3) “endometrial,” “endometrium,” “endocervical,” “endocervix,” “uterine,” or “uterus.” The resulting pathology reports were manually reviewed, identifying 43 additional candidate atypical uterine polyps diagnosed between 2005 and 2020.
Clinical and morphologic parameters
All available hematoxylin and eosin (H&E)-stained slides were reviewed. For study inclusion, a polyp needed to show at least one of the following atypical features, albeit insufficiently qualitatively or quantitatively developed for diagnosis of adenosarcoma: (1) abnormal architecture (early or focal phyllodiform growth, rigid cystic glands, and/or intraluminal papillary projections); (2) abnormal periglandular stroma (mild or focal periglandular stromal cuffing and/or subtle subepithelial condensation); (3) stromal atypia (characterized by enlarged hyperchromatic nuclei with smudged chromatin, as previously described34). Stromal mitotic count was also noted, with >2 mitoses per 10 high-power fields (hpf; 40x objective, field diameter 0.55 mm, 10 hpf = 2.4 mm2) considered increased; however, increased mitotic count in the absence of other atypical features was insufficient for study inclusion. Out of 43 candidate atypical polyps, 34 (20 in-house, 14 consults) met morphologic inclusion criteria.
Clinical parameters, including patient age, relevant medical history, presenting signs/symptoms, site and sampling technique for initial diagnostic specimen, follow-up type and interval, subsequent uterine pathology, and outcome were obtained from the electronic medical record. Two types of follow-up were considered: (1) “pathological follow-up,” defined by repeat uterine tissue sampling or hysterectomy with pathological examination; and (2) “clinical follow-up,” defined by ongoing medical observation, providing information about the patient’s clinical disease status and survival. Polyp size was determined by clinical, radiographic, and/or pathologic examination. Morphologic parameters evaluated in each polyp included individual atypical features (see inclusion criteria, above), the extent of polyp involvement by atypical features, and epithelial differentiation. Morphologic assessment for study inclusion was performed by two gynecological pathologists (B.E.H. and M.R.N. for 29 previously reported polyps; D.B.C. and M.R.N. for 34 novel polyps).
Immunohistochemistry
Immunohistochemical assay conditions are described in SupplementaryTable 1. Twenty-eight polyps were evaluated by HMGA2 immunohistochemistry. Six polyps were excluded due to insufficient tissue. Two polyps with MDM2 amplification by FISH (see below) were evaluated by MDM2 immunohistochemistry. HMGA2 and MDM2 overexpression were defined by strong diffuse nuclear staining. Twenty-one polyps with NGS data were evaluated by ER and PR immunohistochemistry. The extent of ER and PR expression in periglandular stromal cells was classified as diffuse (≥90%) or patchy (<90%).
Fluorescence in situ hybridization
HMGA2 fluorescence in situ hybridization (FISH) was performed on 8 polyps with positive HMGA2 immunostaining, and MDM2 FISH was performed in both polyps with HMGA2 amplification. FISH analysis was performed on 4 µm tissue sections and evaluated using laboratory-developed, clinically validated dual-color break-apart HMGA2 probes specific for the 5′ and 3′ regions of 12q14.3, and with MDM2 at 12q15 and D12Z3 (chromosome 12 alpha satellite probe) at 12p11.1-q11.1 (Abbott Molecular; Abbott Park, IL, USA). Labeling and hybridization of the probes were performed according to standard laboratory protocols. FISH results were evaluated in tumor areas marked by a gynecological pathologist (D.B.C.). Copy number gain was defined by 3 or 4 gene copies, and amplification was defined by ≥5 gene copies.
Next-generation sequencing
Twenty-three polyps were analyzed by hybrid capture NGS on the 447-gene OncoPanel platform35. Each case was annotated for point mutations, small insertions and deletions, and copy number alterations. Variants were filtered to remove technical artifacts, synonymous variants, and any population variants at >0.1% frequency in the Genome Aggregation Database (https://gnomad.broadinstitute.org/), and the remaining pass-filter variants were assessed for likely pathogenicity by a molecular pathologist (L.M.S.). Copy number alterations were determined with an internally developed pipeline (RobustCNV). Copy number of ≥6 was defined as “amplification,” whereas lesser copy number gains were classified as “gains.” HMGA2 was not included in the gene panel, though chr 12q13-15 (including the chr 12q14.3 band containing HMGA2) was tiled for copy number calling. Accordingly, HMGA2 copy number alterations were inferred from alterations in flanking genes. NGS Sequencing data from this study are publicly available through the AACR Genie database.
Results
Clinical findings and outcomes
Clinical data are summarized in Table 1. Detailed clinical and morphologic data on all patients are in Supplementary Table 2. The final cohort included 63 (58 primaries, 5 residual/recurrent) atypical uterine polyps from 58 women, ranging from 23–75 (mean, 50) years old. Twenty-nine women (50%) presented with abnormal uterine bleeding (including 12 with postmenopausal bleeding), 21 (36%) presented with a polyp on clinical examination or ultrasound, and 8 (14%) were discovered incidentally on workup for other indications. Forty-seven (81%) women had endometrial polyps, compared with 11 endocervical. Forty-two (72%) were diagnosed by endometrial biopsy, curettage, and/or polypectomy, 15 (26%) by endocervical curettage and/or polypectomy, 3 (5%) in hysterectomy specimens, and 1 was spontaneously passed.
Follow-up was available for 47 patients (Fig. 1). (All 11 cases without follow-up information came from authors’ consultation files.) In 3 women, atypical uterine polyp was diagnosed at hysterectomy, obviating further pathological follow-up. Of the remaining 44 women at risk for residual or recurrent disease, 8 (18%) had no further tissue sampling, whereas 36 (82%) had follow-up pathological examination, including curettage in 28 (78%) and hysterectomy in 8 (22%). The median time from the initial diagnosis to first follow-up pathological examination was 3 months (range, 0.3–104 months; interquartile range, 1.1–6.9 months).
Among 36 women undergoing follow-up pathological examination, 16 (44%) had no lesion identified, 16 (44%) had a morphologically conventional polyp, and 4 (11%) had residual or recurrent atypical uterine polyp (including 1 patient with 2 recurrences). The 4 instances of residual/recurrent atypical uterine polyp were diagnosed at 1, 5, 7, and 11 months after initial diagnosis (see supplementary information 1). Four more women underwent a hysterectomy after initial follow-up curettage (that showed no lesion in 1, banal polyp in 2, and atypical polyp in 1), for a total of 12/44 women (27%) undergoing hysterectomy subsequent to a diagnosis of atypical uterine polyp.
No patient developed uterine adenosarcoma over median pathological follow-up of 7.5 months (range, 0.7–115 months; interquartile range, 3–14 months) and median clinical follow-up of 150 months (range, 2–350 months; interquartile range, 55–186 months). No patient received adjuvant radiation or chemotherapy. At last follow-up, 46 women were alive with no evidence of disease, and 1 woman had died of pancreatic ductal adenocarcinoma 25 months after diagnosis of atypical uterine polyp.
Gross and microscopic findings
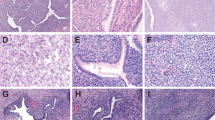
Pathological findings in the 58 primary atypical uterine polyps are summarized in Table 2. Representative photomicrographs are shown in Fig. 2. Clinicopathologic features of the 5 residual/recurrent atypical polyps are detailed in Supplementary Information 1. Due to frequent specimen fragmentation, size could be determined in only 21 polyps (median, 2.4 cm, range, 0.5–5.8 cm). Abnormal architecture was present in 46 (79%), including early or focal phyllodiform architecture (n = 32) and/or rigid cystic glands (n = 29). Mild or focal periglandular stromal cuffing was seen in 44 (76%), including foci of subtle subepithelial stromal condensation in 13. Mitoses ranged from 0–16 (median 1, mean 2) per 10 hpf, with greater than 2 mitoses per 10 hpf in 16 (28%). Mitotic activity was higher in endometrial polyps (median, 1 per 10 hpf; range, 0–16) than in endocervical polyps (median, 0 per 10 hpf, range 0–4), though the difference was not statistically significant (P = 0.19, Mann–Whitney U test, two-tailed). (Out of 7 endometrial polyps with ≥6 stromal mitoses per 10 hpf, 6 also harbored epithelial mitoses and proliferative-pattern glands, and 1 patient was on tamoxifen for prior breast cancer). Stromal atypia was seen in 10 (17%), characterized by enlarged, irregular, hyperchromatic nuclei with smudged chromatin, single nucleoli, occasional nuclear pseudo inclusions, and (in one case) multinucleation. No heterologous elements were identified. Fourteen (24%) polyps showed 1 atypical feature (including 7 endometrial polyps with stromal atypia only), 30 (52%) showed 2 atypical features, and 14 (24%) showed 3 atypical features. No polyp showed all 4 atypical features. It is emphasized that the atypical features in these polyps though worrisome, were insufficiently developed to warrant diagnosis of adenosarcoma on consensus morphologic review by subspecialty gynecologic pathologists.
A Focal phyllodiform growth with periglandular stromal cuffing in an edematous stromal background (Case 22, 20x). B Focal phyllodiform growth with subtle periglandular stromal cuffing (Case 10, 20x). C Focal phyllodiform growth and blunt papillary surface projections, without significant periglandular stromal changes (Case 6, 20x). D Periglandular stromal cuffing with blunt intraglandular papillary projections, but without overt phyllodiform architecture (Case 13, 40x). E Subtle subepithelial stromal condensation (Case 14, 400x). F Rigid dilated cystic glands (center) and blunt surface papillary projections (left), with subtly increased periglandular stromal cellularity (Case 10, 20x). G Increased stromal mitoses (yellow arrow) were, in most cases, seen concurrent to increased epithelial mitoses (green arrow) (Case 20, 400x). H Moderate stromal cytologic atypia (Case 11, 200x).
No clinical or morphologic features—including age, number, type, or distribution of atypical features; or interval from the initial diagnosis to pathologic follow-up—were significantly associated with the presence of residual/recurrent atypical polyp on follow-up sampling. In the 4 patients with residual/recurrent polyp on follow-up sampling, the residual/recurrent polyp in each case showed the same atypical morphologic feature(s) noted in the initial atypical polyp. In 2 of 4 cases, the atypical morphologic features more extensively involved the residual/recurrent polyp compared with the initial polyp specimen. However, complete histologic examination of both residual/recurrent polyps (both from hysterectomy specimens) showed that the atypical morphologic features still fell qualitatively short of the threshold for diagnosis of adenosarcoma.
Immunohistochemical and molecular findings
Immunohistochemical and molecular findings are represented in Fig. 3. Point mutations are detailed in Table 3. In total, HMGA2 immunohistochemistry was performed on 28 polyps with available tissue. Successful molecular profiling was performed on 22 (21 primary and 1 residual/recurrent) atypical uterine polyps, including NGS alone in 17, FISH alone in 1, and both assays in 4.
Stromal cells in 8 (29%) of 28 polyps showed immunohistochemical HMGA2 overexpression (Figs. 4, 5). In polyps only partially involved by atypical morphologic features, HMGA2 has overexpressed in the morphologically unremarkable as well as the morphologically atypical foci. The glandular component of all polyps showed negative to patchy weak HMGA2 expression, interpreted as nonspecific7.
A Subtle periglandular stromal hypercellularity. B Scattered atypical nuclei with hyperchromatic smudged chromatin. C Subtle subepithelial stromal condensation (C, upper left, arrow) (200x). D Stromal cell HGMA2 overexpression by immunohistochemistry (200x). E Stromal cell MDM2 overexpression by immunohistochemistry (200x). F Breakapart HMGA2 FISH shows HMGA2 amplification without evidence of HGMA2 rearrangement. G MDM2 FISH shows MDM2 amplification, without polysomy of chr 12.
A Moderate stromal cytologic atypia (200x). B Stromal cell HGMA2 overexpression by immunohistochemistry (200x). C Stromal cell MDM2 overexpression by immunohistochemistry (200x). D Breakapart HMGA2 FISH shows HMGA2 amplification without evidence of HGMA2 rearrangement. E MDM2 FISH shows MDM2 amplification. F, G NGS copy number profiling shows a largely quiet genome with a single amplification on chr 12, encompassing chr 12q13-15 (NAB2, STAT6, GLI1, CDK4, HMGA2, and MDM2). Note that the HMGA2 locus is indicated in parentheses, as the NGS panel tiles chr 12q14.3 but not HMGA2.
HMGA2 FISH was successfully performed in 5 primary atypical uterine polyps with immunohistochemical HMGA2 overexpression (3 polyps failed hybridization − 1 each from 2010, 2011, and 2017). FISH confirmed HMGA2 copy number gain (n = 2) or amplification (n = 2) in 4 of 5 polyps (Figs. 4, 5, Supplementary Table 3), and identified no apparent copy number alteration in the remaining polyp. No HMGA2 rearrangements were identified. MDM2 FISH was performed in both polyps with HMGA2 amplification and confirmed co-amplification of MDM2 and HMGA2 in both. MDM2 overexpression was confirmed in both by immunohistochemistry (Figs. 4, 5, Supplementary Table 3).
NGS was successfully performed in 20 primary atypical uterine polyps and 1 residual/recurrent atypical polyp (2 polyps failed quality control − 1 each from 2010 and 2019). NGS identified 0-5 (median, 1) copy number alterations per polyp. Three recurrent copy number profiles were identified:
-
1.
Chr 12q13-15 gain or amplification (3-8 copies) was seen in 6 (27%) of 22 polyps (including 1 residual/recurrent atypical polyp) and varied in size from chr 12q13.2-q15 amplification (polyp #1; see Fig. 3) to isolated chr 12q14.3 (HMGA2) amplification (#4; confirmed by FISH). FISH and NGS yielded concordant HMGA2 copy number calls in 3 of 4 polyps evaluated by both techniques (Fig. 5), with 1 discordant call attributed to inadequate NGS coverage of a small chr 12q14.3 amplicon. Of 6 polyps with positive HMGA2 IHC and molecular profiling, HMGA2 gain or amplification was present in 4 and absent in 2. MDM2 FISH and NGS showed concordant MDM2 amplification in 2 of 2 cases evaluated by both techniques. Supplementary Table 3 provides detailed correlation of immunohistochemical, FISH, and NGS results for HMGA2 and MDM2.
One polyp (#1) with chr 12q13-15 amplification (estimated 8 copies) also showed low-level whole-arm gain of chr 1q (estimated 1-2 copy gain), whole-chromosome loss of chr 7 (estimated 1 copy deletion), amplification of chr 12q24.12-13 (estimated 8 copies), amplification of chr 15p24.1-26.1 (estimated 12-25 copies), and amplification of chr 18q21.33 (BCL2; estimated 21 copies). On consensus morphologic re-review, this polyp showed features considered borderline between atypical uterine polyp and adenosarcoma (Supplementary Fig. 1).
-
2.
Low-level copy number gain (estimated 2.5-5 copies) of chr 6q25.1 (ESR1) was detected in 9 primary and 1 residual/recurrent atypical uterine polyps across 3 sequencing runs. There was no difference in immunohistochemical expression of ER or PR in the stroma of polyps with versus without ESR1 gain (Supplementary Fig. 2). One polyp (#6) showed ESR1 gain as well as chr 14q32.12 (SERPINA1) amplification (estimated 6 copies). One pair of primary (#5a) and residual/recurrent (#5b) polyps showed both ESR1 gain and chr 12q14.1 (CDK4; estimated 3 copies) gain.
-
3.
Seven polyps showed no significant copy number alterations. Two of these (#15, 17) showed immunohistochemical HMGA2 overexpression, suggesting that this pathway may be activated by alternative mechanisms in a subset of atypical polyps with apparently silent copy number profiles. All 6 polyps with chr 12q13-15 gain or amplification and both polyps with immunohistochemical HMGA2 overexpression but no apparent copy number gains were endometrial, suggesting that HMGA2 pathway activation may be site-specific. Both endocervical and endometrial polyps were represented in the molecular subgroups with chr 6q25.1 (ESR1) copy number gain and with no significant copy number alterations.
Two polyps showed unique copy number profiles, not belonging to the above-defined subgroups. One polyp (#13, representing the patient’s second recurrence of an atypical endocervical polyp) showed copy number gains at chr 8p12 (NRG; estimated 3-4 copies) and chr 10q11.21-23 (RET, ERCC6; estimated 3 copies). The other (#14) showed copy number loss at chr 11q21 (MRE11A; partial 1 copy deletion, spanning the 5' end of the gene to exon 10), as well as a pathogenic KRAS mutation. No polyp had alterations associated with aggressive clinical behavior in adenosarcoma, including MYBL1 amplification, CDKN2A deletion, BAP1 deletion, TP53 deletion or mutation, RB1 deletion, ATRX mutation, or DICER1 mutation.
Among 20 primary atypical uterine polyps sequenced by NGS, median tumor mutational burden was 3.04 (range, 0.76-8.37) mutations/Mb. Pathogenic point mutations in oncogenes and/or tumor suppressor genes were identified in 13 polyps (see Table 3). Of 7 polyps with stromal atypia only, 4 underwent successful molecular profiling: 2 (#11, 12) showed ESR1 gains only, 1 (#2) showed chr 12q13-15 amplification only, and 1 (#4) showed ESR1 gain and chr 12q13-15 amplification. The 2 residual/recurrent lesions profiled by NGS (#5b, 13) had unique molecular profiles, precluding identification of molecular features associated with recurrence. One pair of matched primary and residual/recurrent polyps (#5a, 5b) showed identical molecular profiles, supporting a clonal relationship.
Discussion
This study comprises related clinicopathologic and molecular components:
-
1.
A comprehensive clinicopathologic profile of 58 women with uterine polyps showing atypical features non-diagnostic of adenosarcoma, expanding upon a prior cohort of 28 cases reported from our institution33.
-
2.
A molecular profile of 22 atypical uterine polyps, using a targeted NGS panel, FISH, and immunohistochemistry to explore the biologic relationship of our atypical uterine polyps to reported molecular features of conventional benign endometrial polyps and uterine adenosarcoma.
Comprehensive clinicopathologic profiling of 58 women with atypical uterine polyps confirms the clinical and morphologic characteristics reported previously33. Although we found a modest (11%) risk of residual/recurrent atypical polyp at short-term follow-up, none of our cases progressed to uterine adenosarcoma, despite long-term follow-up (median, 12.5 years) and conservative (i.e., non-hysterectomy) management in two-thirds of patients. Architectural abnormalities are the most common atypical morphologic feature, closely followed by abnormalities of periglandular stroma, with these two features often co-occurring. Increased mitoses are seen in a minority, principally in endometrial polyps from women in the proliferative phase of the endometrial cycle, and were never the sole atypical feature. Atypical uterine polyps (median, 2.4 cm; range, 0.5–5.8 cm) were, on average, significantly smaller than adenosarcomas (mean, 6.3 cm; range, 2–12 cm) seen at our institution23.
Molecular profiling identifies three principal subgroups of atypical uterine polyps: (1) atypical polyps with chr 12q13-15 gain or amplification, (2) atypical polyps with chr 6q25.1 gain, and (3) atypical polyps with no detectable copy number alterations. Our atypical polyps shared multiple recurrent molecular alterations with low-grade uterine adenosarcoma, but they harbored fewer total copy number alterations per polyp (range, 0–5; median, 1) than previously reported in low-grade adenosarcoma (range, 0–12; median, 3)23. Together, these findings indicate that at least some atypical uterine polyps may represent incipient uterine adenosarcomas.
HMGA2 overexpression is a shared feature of morphologically conventional endometrial polyps, atypical uterine polyps, and adenosarcomas. Variably sized gains or amplification of chr 12q13-15 (harboring HMGA2) were identified in 5 (24%) of 21 primary atypical uterine polyps, falling within the 24-33% prevalence of this alteration in uterine adenosarcoma23,27,30. Chr 12q13-15 amplifications occur in the full clinical-morphologic spectrum of adenosarcomas, including tumors with and without sarcomatous overgrowth and tumors with and without local or distant recurrence23,26,27,29,36. In contrast, chr 12q13-15 amplification is exceptionally rare in conventional endometrial polyps14, that instead harbor chr 12q14.1 (HMGA2) rearrangement6,7,10,11,12,13. Furthermore, approximately 30% of banal endometrial polyps7, 10% of atypical uterine polyps, and 25% of adenosarcomas29 show immunohistochemical HMGA2 overexpression in the absence of HMGA2 rearrangement or amplification, which may result from dysregulation of key miRNAs or from HMGA2 truncation in exon 3, leading to evasion of miRNA-mediated gene silencing37,38,39.
One atypical uterine polyp (#1) harbored chr 12q13-15 amplification alongside a whole-arm gain of chr 1q, chr 12q24.12-24.13 amplification, chr 15q24.1-26.1 amplification, and chr 18q21.33 amplification. Three of these copy number alterations have been reported in adenosarcoma: Chr 1q gain has been reported in adenosarcomas with low-grade and high-grade morphology, with and without sarcomatous overgrowth, and with and without local or distant recurrence23,27,30. Chr 12q24.12-24.13 amplification has been reported in 10–40% of adenosarcomas, likewise spanning the spectrum of morphologic grade and clinical behavior23,30. And high-level amplification of chr 18q21.33 (BCL2) was found in ~25% of adenosarcomas in one series30. Tellingly, on consensus morphologic re-review, this lesion was difficult to classify, with features worrisome but indeterminant for adenosarcoma. The concordance of particularly worrisome morphologic features and multiple adenosarcoma-like molecular alterations further supports a biological continuum between atypical uterine polyp and adenosarcoma.
Chr 6q25.1 copy number gain (including ESR1, encoding the estrogen receptor) was detected in 9 (45%) of 20 primary atypical uterine polyps sequenced by NGS. Chr 6q24.3-q25.3 gains were reported in 2 of 10 adenosarcomas (both morphologically low-grade, without recurrence) studied by whole-genome sequencing in one series30, suggesting that this subset of atypical uterine polyps may also be molecularly related to adenosarcoma. An adenosarcoma with ESR1-NCOA3 fusion has also been reported36. Chr 6q25.1 copy number alterations have not been described in endometrial polyps15.
Seven (33%) of 21 primary atypical uterine polyps showed no copy number alterations. Likewise, minor subsets of morphologically banal endometrial polyps6 and low-grade uterine adenosarcomas23,28 also show “quiet genomes,” without significant copy number alterations. In uterine adenosarcoma, absence of copy number alterations has been associated with indolent behavior. In one study of 18 adenosarcomas, all 4 lesions with no copy number alterations lacked sarcomatous overgrowth and none recurred23. Conversely, copy number alterations are significantly greater in adenosarcomas with high-grade stromal atypia and sarcomatous overgrowth23,29,36. In our cohort, 2 of 6 atypical uterine polyps with at least 1 copy number alteration had residual/recurrent lesion on follow-up sampling, compared with 0 of 6 polyps with no copy number alterations, suggesting that a quiet genome may be associated with more indolent behavior in this setting, as well. Given the rarity of residual/recurrent lesion on follow-up, a very large series of atypical uterine polyps with comprehensive molecular analysis and robust clinical follow-up would be needed to confirm this impression.
Beyond these 3 recurrent molecular subgroups, 2 atypical uterine polyps showed unique copy number profiles. One polyp harbored chr 11q21 (MRE11A) loss, which was reported in 2 of 10 adenosarcomas studied by whole-genome sequencing30. A second polyp harbored low-level gains in chr 8p12 (NRG) and chr 10q11.21-23 (RET, ERCC6)- although neither of these alterations has been reported in adenosarcoma, one karyotype-based study found frequent chr 8 alterations in uterine adenosarcoma28.
In this series, 12 (60%) of 20 primary atypical uterine polyps sequenced by NGS harbored pathogenic point mutations (Fig. 3, Table 3), including recurrent point mutations seen in adenosarcoma. AKT1 p.E17K hotspot mutation was identified in 1 atypical polyp and has been reported in 2 low-grade adenosarcomas, with PTEN/AKT/PIK3CA pathway mutations in 26–72% of adenosarcomas overall23,27. ERBB2 and APC missense mutations were each identified in 1 atypical uterine polyp, and have likewise been reported in adenosarcoma23. A SMARCE1 nonsense mutation was identified in 1 atypical uterine polyp with a complex copy number profile. Although SMARCE1 mutations have not been reported in adenosarcoma, loss-of-function mutations in other SWI/SNF proteins have, including SMARCA4 nonsense mutation23 and SMARCB1 frameshift mutation with loss of heterozygosity27. Despite these shared alterations, atypical uterine polyps have a significantly lower tumor mutational burden (3.1 mutations/Mb) than adenosarcomas (~9.6 mutations/Mb)23.
Conversely, KRAS, ARHGAP35, and PPP2R1A mutations detected in our samples have also been reported in morphologically banal endometrial polyps. In a study of 4 endometrial polyps by whole-exome sequencing with matched germline DNA, 1 polyp each harbored a mutation in PPP2R1A and ARHGAP35, and KRAS mutations were detected in 2 of 4 polyps and in an additional 14 of 31 polyps studied by validation PCR (16/35 total, 46%)15. However, we favor these oncogene mutations in our atypical polyps to represent incidentally captured alterations in the epithelial compartment, as recent evidence indicates that the endometrial epithelium accumulates pathogenic mutations in cancer-related genes (including ARHGAP35, PPP2R1A, KRAS, ERBB3, ERBB2, and FBXW7) from an early age17, and laser capture microdissection-based mitochondrial DNA profiling in adenosarcoma has shown no clonal relationship between the epithelial and stromal compartments27.
Although the majority of uterine adenosarcomas show low-grade morphology and relatively indolent behavior18,22,24, a minority show high-grade morphologic features associated with more aggressive clinical behavior18,22,25,26 and recurrent molecular alterations. Sarcomatous overgrowth is associated with increased copy number losses23,29, and aggressive clinical behavior is associated with amplification of MYBL1 amplification23 and deletion of CDKN2A23,29,36, BAP123,26,27,30,36, TP5329, and RB123,29. Additionally, high-grade stromal atypia, sarcomatous overgrowth, and heterologous rhabdomyosarcomatous differentiation are linked to point mutations in ATRX, DICER1, and TP5323,26,27,36. In keeping with their low-grade morphologic features, no atypical uterine polyp harbored these “high-grade” alterations. It is noteworthy, however, that high-grade and low-grade adenosarcomas share common molecular features, particularly chr 12q13-15 amplification, suggesting that atypical uterine polyps, low-grade adenosarcoma, and high-grade adenosarcoma represent a molecular continuum, rather than discrete biologic entities. This, in turn, raises the possibility that, if morphologic or molecular-based risk stratification for these lesions could be refined, a subset of lesions currently diagnosed as adenosarcoma might be safely managed conservatively – a particular benefit to young women wishing to preserve fertility.
Some pathologists may diagnose a subset of our atypical polyps as uterine adenofibroma – a reportedly rare neoplasm comprising benign glands and stroma32. Although we do not use this diagnosis at our institution, proposed definitions of uterine adenofibroma18,19,32,40 overlap somewhat with the criteria for our atypical uterine polyps. Furthermore, uterine adenofibroma and adenosarcoma without sarcomatous overgrowth have similar immunoprofiles19, and adenofibromas may rarely invade the myometrium40 or recur after hysterectomy19. These findings suggest that a subset of adenofibromas represent subtle or incipient adenosarcomas, akin to our atypical uterine polyps, and indicate that such lesions may occasionally be clinically aggressive, despite universally benign behavior in our series. This study provides a basis for molecular comparison to lesions diagnosed as adenofibroma, which could further elucidate the relationship of these entities.
This study has certain limitations. First, diagnosis of this sort of atypical uterine polyp is not codified, and there is likely variability in diagnostic criteria, nomenclature, and management. The distinction of subtle atypical polyps from conventional benign endometrial polyps is challenging: on morphologic re-review, we considered nearly one-quarter of polyps initially diagnosed as “atypical uterine polyp” to instead represent banal endometrial polyps. On the other end of the spectrum, distinction from subtle low-grade adenosarcoma is also challenging, and the molecular overlap between atypical polyps and low-grade adenosarcoma suggests that these entities exist on a continuum. Second, molecular testing could only be performed on a subset of atypical polyps, potentially limiting our detection of certain molecular findings. Targeted NGS may also miss some pathogenic alterations, as well as balanced structural variants, which may play a role in adenosarcoma pathogenesis28,30. Also, our NGS panel tiles chr 12q14.3, but HMGA2 is not among the sequenced genes; accordingly, NGS-detected HMGA2 copy number gains are inferred, albeit with a high degree of confidence. The low-level ESR1 copy number gains detected in this study also warrant orthogonal validation. Finally, molecular studies of conventional benign endometrial polyps are principally based on karyotyping, and large NGS-based studies of conventional benign endometrial polyps are lacking, which complicates comparison with our molecular findings. We cannot exclude that a subset of morphologically banal endometrial polyps harbors some of the alterations described here.
In summary, uterine polyps with atypical features worrisome for, but not diagnostic of, adenosarcoma share multiple molecular alterations with low-grade adenosarcomas, providing evidence that these two entities exist on a biological spectrum and that atypical uterine polyps, in some cases, likely represent incipient adenosarcoma. However, our clinicopathologic data confirm that these atypical uterine polyps are associated with an extremely low risk of a malignant clinical course.
We considered alternate terminology for these lesions, out of concern that “atypical uterine polyp” could be confused with “atypical polypoid adenoma” or “atypical endometrial hyperplasia,” particularly among non-pathologists. However, we ultimately felt that the “atypical” designation was important to ensure proper clinical attention to this diagnosis. In individual instances, the terms “atypical endometrial polyp” and “atypical endocervical polyp” may be used for greater specificity. To avoid clinical misinterpretation, we have omitted the word “adenosarcoma” or “adenosarcoma-like” from the proposed terminology.
In routine practice, we recommend a diagnosis of “atypical uterine polyp” for endocervical or endometrial polyps with abnormal architecture (subtle or focal phyllodiform growth, rigid cystic glands, and intraluminal papillary projections) and/or subtle periglandular stromal cuffing and/or stromal atypia. Stromal mitoses should be considered in the context of the background endometrium, as increased mitoses may be seen in a proliferative background. Increased mitoses alone do not warrant a diagnosis of atypical uterine polyp. Atypical morphologic findings may be focal or diffuse, but they must be mild in degree, falling short of the diagnostic threshold for adenosarcoma. Atypical uterine polyp is, at present, a fundamentally morphologic diagnosis. Available evidence does not identify any immunohistochemical or molecular assays that predict behavior in atypical uterine polyps or that meaningfully distinguish these lesions from subtle adenosarcomas, and no ancillary studies are currently advocated for routine use. Given the rarity of these lesions, it is appropriate to include a diagnostic comment communicating their biological nature and expected clinical behavior. We advocate a low threshold for seeking expert consultation.
Our findings suggest that atypical uterine polyps can be managed conservatively. Nonetheless, clinical follow-up with repeat tissue sampling is advised given their apparent biologic overlap with adenosarcoma. Given that all 4 instances of recurrent atypical uterine polyp in this series were discovered within 1 year, repeat sampling at 6–12 months appears prudent, though earlier sampling is advised if there is clinical suspicion for recurrence. Further studies are warranted to develop more robust morphologic and molecular risk stratification models for uterine stromal neoplasms, to more precisely determine whether a subset of lesions presently diagnosed as adenosarcoma might also be safely managed without hysterectomy.
Data availability
NGS Sequencing data from this study are publicly available through the AACR Genie database. Detailed clinicopathologic data for patients undergoing molecular characterization are available in Supplementary Table 2.
References
Fletcher, J. A., Pinkus, J. L., Lage, J. M., Morton, C. C. & Pinkus, G. S. Clonal 6p21 rearrangement is restricted to the mesenchymal component of an endometrial polyp. Genes Chromosomes Cancer 5, 260–263 (1992).
Caroti, S. & Siliotti, F. Cervical polyps: A colpo-cyto-histological study. Clin. Exp. Obstet. Gynecol. 15, 108–115 (1988).
Golan, A., Ber, A., Wolman, I. & David, M. P. Cervical polyp: Evaluation of current treatment. Gynecol. Obstet. Invest. 37, 56–58 (1994).
Van Bogaert, L. J. Clinicopathologic findings in endometrial polyps. Obstet. Gynecol. 71, 771–773 (1988).
Reslová, T., Tosner, J., Resl, M., Kugler, R. & Vávrová, I. Endometrial polyps. A clinical study of 245 cases. Arch Gynecol. Obstet. 262, 133–139 (1999).
Dal Cin, P. et al. Four cytogenetic subgroups can be identified in endometrial polyps. Cancer Res. 55, 1565–1568 (1995).
Tallini, G. et al. HMGI-C and HMGI(Y) immunoreactivity correlates with cytogenetic abnormalities in lipomas, pulmonary chondroid hamartomas, endometrial polyps, and uterine leiomyomas and is compatible with rearrangement of the HMGI-C and HMGI(Y) genes. Lab. Invest. 80, 359–369 (2000).
Vanni, R., Marras, S., Andria, M. & Faa, G. Endometrial polyps with predominant stromal component are characterized by a t(6;14)(p21;q24) translocation. Cancer Res. 55, 31–33 (1995).
Speleman, F. et al. Is t(6;20)(p21;q13) a characteristic chromosome change in endometrial polyps? Genes Chromosomes Cancer 3, 318–319 (1991).
Walter, T. A. et al. inv(12)(p11.2q13) in an endometrial polyp. Cancer Genet. Cytogenet. 41, 99–103 (1989).
Bol, S. et al. An endometrial polyp with a rearrangement of HMGI-C underlying a complex cytogenetic rearrangement involving chromosomes 2 and 12. Cancer Genet. Cytogenet. 90, 88–90 (1996).
Vanni, R. et al. Endometrial polyp: another benign tumor characterized by 12q13-q15 changes. Cancer Genet. Cytogenet. 68, 32–33 (1993).
Hennig, Y., Wanschura, S., Deichert, U., Bartnitzke, S. & Bullerdiek, J. Rearrangements of the high mobility group protein family genes and the molecular genetic origin of uterine leiomyomas and endometrial polyps. Mol. Hum. Reprod. 2, 277–283 (1996).
Dal Cin, P. et al. Amplification and expression of the HMGIC gene in a benign endometrial polyp. Genes Chromosomes Cancer 22, 95–99 (1998).
Takeda, T. et al. Mutations of RAS genes in endometrial polyps. Oncol Rep. 42, 2303–2308 (2019).
Hachisuga, T. et al. K-ras mutation in tamoxifen-related endometrial polyps. Cancer 98, 1890–1897 (2003).
Moore, L. et al. The mutational landscape of normal human endometrial epithelium. Nature 580, 640–646 (2020).
Clement, P. B. & Scully, R. E. Mullerian adenosarcoma of the uterus: a clinicopathologic analysis of 100 cases with a review of the literature. Hum. Pathol. 21, 363–381 (1990).
Gallardo, A. & Prat, J. Mullerian adenosarcoma: a clinicopathologic and immunohistochemical study of 55 cases challenging the existence of adenofibroma. Am. J. Surg. Pathol. 33, 278–288 (2009).
Manoharan, M., Azmi, M. A. N., Soosay, G., Mould, T. & Weekes, A. R. Mullerian adenosarcoma of uterine cervix: Report of three cases and review of literature. Gynecol. Oncol. 105, 256–260 (2007).
Chin, P.-S., Chia, Y.-N., Lim, Y.-K. & Yam, K.-L. Diagnosis and management of Müllerian adenosarcoma of the uterine cervix. Int. J. Gynaecol. Obstet. 121, 229–232 (2013).
Verschraegen, C. F. et al. Clinicopathologic analysis of mullerian adenosarcoma: The M.D. anderson cancer center experience. Oncol. Rep. 5, 939–944 (1998).
Howitt, B. E. et al. Targeted genomic analysis of Müllerian adenosarcoma. J. Pathol. 235, 37–49 (2015).
Brooks, S. E., Zhan, M., Cote, T. & Baquet, C. R. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989-1999. Gynecol. Oncol. 93, 204–208 (2004).
Clement, P. B. Müllerian adenosarcomas of the uterus with sarcomatous overgrowth. A clinicopathological analysis of 10 cases. Am. J. Surg. Pathol. 13, 28–38 (1989).
Hodgson, A., Amemiya, Y., Seth, A., Djordjevic, B. & Parra-Herran, C. High-grade müllerian adenosarcoma: Genomic and clinicopathologic characterization of a distinct neoplasm with prevalent TP53 pathway alterations and aggressive behavior. Am. J. Surg. Pathol. 41, 1513–1522 (2017).
Piscuoglio, S. et al. Uterine adenosarcomas are mesenchymal neoplasms. J. Pathol. 238, 381–388 (2016).
Howitt, B. E., Dal Cin, P., Nucci, M. R. & Quade, B. J. Involvement of chromosome 8 in müllerian adenosarcoma. Int. J. Gynecol. Pathol. 36, 24–30 (2017).
Lee, J.-C. et al. Genomewide copy number analysis of Müllerian adenosarcoma identified chromosomal instability in the aggressive subgroup. Mod. Pathol. 29, 1070–1082 (2016).
Ban, Y. et al. Whole-Genome Sequencing and Target Validation Analysis of Müllerian Adenosarcoma: A Tumor With Complex but Specific Genetic Alterations. Front Oncol 10, 538 (2020).
Kerner, H. & Lichtig, C. Müllerian adenosarcoma presenting as cervical polyps: A report of seven cases and review of the literature. Obstet. Gynecol. 81, 655–659 (1993).
Zaloudek, C. J. & Norris, H. J. Adenofibroma and adenosarcoma of the uterus: a clinicopathologic study of 35 cases. Cancer 48, 354–366 (1981).
Howitt, B. E., Quade, B. J. & Nucci, M. R. Uterine polyps with features overlapping with those of Müllerian adenosarcoma: A clinicopathologic analysis of 29 cases emphasizing their likely benign nature. Am. J. Surg. Pathol. 39, 116–126 (2015).
Tai, L. H. & Tavassoli, F. A. Endometrial polyps with atypical (bizarre) stromal cells. Am. J. Surg. Pathol. 26, 505–509 (2002).
Sholl, L. M. et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI. Insight. 1, e87062 (2016).
Bean, G. R., Anderson, J., Sangoi, A. R., Krings, G. & Garg, K. DICER1 mutations are frequent in müllerian adenosarcomas and are independent of rhabdomyosarcomatous differentiation. Mod. Pathol. 32, 280–289 (2019).
Agostini, A. et al. Genomic imbalances are involved in miR-30c and let-7a deregulation in ovarian tumors: implications for HMGA2 expression. Oncotarget. 28, 21554–21560 (2017). 8.
Agostini, A. et al. A novel truncated form of HMGA2 in tumors of the ovaries. Oncol. Lett. 12, 1559–1563 (2016).
Nagaishi, M. et al. High HMGA2 expression without gene rearrangement in meningiomas. Neuropathology 40, 540–545 (2020).
Clement, P. B. & Scully, R. E. Müllerian adenofibroma of the uterus with invasion of myometrium and pelvic veins. Int. J. Gynecol. Pathol. 9, 363–371 (1990).
Funding
Dr. Chapel’s work is supported by the Ovarian Cancer Research Alliance [Ann Schreiber Mentored Investigator Award; grant number 650320]. The authors have no other financial disclosures or conflicts of interest.
Author information
Authors and Affiliations
Contributions
DBC and MRN performed study concept and design, all authors performed development of methodology and writing, review and revision of the paper, all authors provided acquisition, analysis and interpretation of data, and statistical analysis. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This study was approved by the institutional review board at Brigham and Women’s Hospital.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chapel, D.B., Howitt, B.E., Sholl, L.M. et al. Atypical uterine polyps show morphologic and molecular overlap with mullerian adenosarcoma but follow a benign clinical course. Mod Pathol 35, 106–116 (2022). https://doi.org/10.1038/s41379-021-00946-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00946-z