Abstract
Several morphologic variants of ALK+ anaplastic large cell lymphoma (ALCL) are recognized. The small cell (SC) and lymphohistiocytic (LH) variants are reported to be associated with poorer outcome in children with ALK + ALCL. In this study of 102 adults with ALK + ALCL, there were 18 (18%) cases of SC and/or LH variants. Patients with SC/LH ALK + ALCL more often had peripheral blood involvement than patients with non-SC/LH neoplasms (60% vs 0%, p = 0.02). There were no other significant differences in clinical features between patients with SC/LH versus non-SC/LH ALK + ALCL. Compared with non-SC/LH cases of ALK + ALCL, neoplasms with SC/LH features were more often positive for CD2 (92% vs. 36%, p = 0.0007), CD3 (81% vs. 15%, p = 0.0001), CD7 (80% vs. 37%, p = 0.03), and CD8 (54% vs. 7%, p = 0.0006). There were no other significant differences in the immunophenotype between SC/LH and non-SC/LH ALK + ALCL cases. The initial chemotherapy regimens and the response rates were similar between patients with ALK + ALCL with SC/LH patterns versus those with non-SC/LH patterns. After a median follow-up of 30.8 months (range, 0.3–208 months), patients with high (>3) International Prognostic Index (IPI) scores had significantly shorter overall survival than patients with low (<3) IPI scores (p = 0.003). However, there was no significant difference in overall or progression-free survival between patients with SC/LH versus non-SC/LH ALK + ALCL (p = 0.99 and p = 0.94, respectively). We conclude that, in adults with ALK + ALCL, SC and LH variants are associated with peripheral blood involvement and a CD8 + immunophenotype with retention of T-cell markers (CD2, CD3, and CD7). However, in contrast with children with ALK + ALCL, SC and LH variants appear to have no impact on prognosis in adults with ALK + ALCL.
Similar content being viewed by others
Introduction
Anaplastic large cell lymphoma (ALCL) is a mature T-cell neoplasm characterized by uniform and strong expression of CD30. Based on anaplastic lymphoma kinase (ALK) expression, ALCL can be further classified into ALK+ and ALK-negative types. ALK positivity results from chromosome translocations or rarely inversions involving ALK at chromosome 2p23 with a variety of partners, most commonly NPM1 at chromosome 5q351. ALK + ALCL typically occurs in children and young adults with a male predominance, accounting for 10–15% of pediatric/adolescent and ~3% of adult non-Hodgkin lymphomas2. Patients with ALK + ALCL have 5-year survival rates of 70–90%, substantially better than patients with ALK-negative ALCL3,4,5,6,7,8.
ALK + ALCLs are morphologically heterogeneous with five morphologic variants being recognized in the current World Health Organization (WHO) classification: common, small cell (SC), lymphohistiocytic (LH), Hodgkin-like, and composite patterns. Approximately 60–70% of ALK + ALCL cases show a common pattern characterized by: (1) cohesive growth pattern commonly involving lymph node sinuses; and (2) large and pleomorphic lymphoma cells with horseshoe-shaped nuclei, dispersed chromatin, single or multiple prominent nucleoli, and abundant cytoplasm (so-called “hallmark” cells). The SC and LH variants represent 15–20% of ALK + ALCL cases and these variants share some morphologic features: smaller lymphoma cells, fewer “hallmark” cells, more variable CD30 expression, and perivascular accumulation of neoplastic cells9,10,11,12,13,14. In addition, the neoplastic cells in the LH variant often resemble those in the SC variant, and there is overlap between these two variants13. Furthermore, the SC and LH patterns can be intermixed within the same neoplasm or occur in different biopsy specimens of the same patient11, suggesting that these two patterns are closely related. In several studies of childhood ALK + ALCL, the SC and/or LH variants have been associated with a poorer clinical outcome12,15,16,17,18. However, the prognostic significance of the SC and LH variants in adults with ALK + ALCL has not been reported in the literature.
In the present study, we examined 102 adults with ALK + ALCL and compared the clinicopathologic features and outcome of patients with the SC and/or LH variants versus other variants.
Materials and methods
Case selection
We searched the database of the Department of Hematopathology at The University of Texas MD Anderson Cancer Center from January 1, 2007, through December 31, 2018 and identified 102 adult (>18 years old) patients who were diagnosed with ALK + ALCL. The diagnosis and subclassification of ALCL were based on criteria specified in the 2016 WHO classification. The diagnosis of ALK + ALCL was confirmed by ALK expression by immunohistochemistry, t(2;5)(p23;q35) by conventional cytogenetics or ALK rearrangement by fluorescence in situ hybridization analysis. Clinical data were obtained by review of medical records. This study was approved by the institutional review board.
Immunophenotypic analysis
Immunohistochemical studies were performed using formalin-fixed, paraffin-embedded tissue sections, either at the time of diagnosis or retrospectively, as described previously19. Immunohistochemical analysis was performed using an automated immunostainer (Leica Bond-Max IHC Stainer, Leica Biosystems Inc, Buffalo Grove, IL, USA). Tissue sections, 4-µm thick, were deparaffinized and underwent heat-induced antigen retrieval using the Bond Max Epitope Retrieval 1 solution (Leica Biosystems Inc) for 15 min. The antibodies used were specific for CD2, CD7, EMA (Leica Biosystems Inc); CD3, CD20, CD43, CD45 (Dako, Carpinteria, CA, USA); CD4 (Cell Marque, Rocklin, CA, USA); CD5 (SP4; LabVision/NeoMarkers, Fremont, CA, USA); CD8, granzyme B (Thermo Fisher, Waltham, MA, USA); ALK (Cell Signaling, Danvers, MA, USA); PAX5 (Transduction Laboratories, San Diego, CA, USA). The Bond Refine Polymer detection system (Leica Biosystems Inc) was used for visualization.
Flow cytometry immunophenotypic analysis was performed on cell suspensions of tissue biopsy specimens or bone marrow aspirates using either a FACScanto II or FACSCalibur cytometer (Becton Dickinson Biosciences, San Jose, CA, USA), as has been described previously20. Lymphocytes were gated for analysis using side scatter versus forward scatter and CD45 expression versus side scatter. The panel of monoclonal antibodies included reagents specific for CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD25, CD30, CD45, CD56, T-cell receptor (TCR) alpha/beta, and TCR gamma/delta (Becton Dickinson Biosciences).
Statistical analysis
Statistical analyses were performed using the GraphPad Prism 8. Fisher’s exact test was used to compare the clinicopathologic features between patients with ALK + ALCL with SC and LH patterns versus other patterns. Overall survival (OS) was calculated from the date of initial diagnosis to the date of death or last follow-up. Progression-free survival (PFS) was calculated from the date of diagnosis to the date of progression/relapse or, if no progression/relapse, the date of death or last follow-up. Survival was analyzed using the Kaplan–Meier method and was compared using the log-rank test. A P value of less than 0.05 was considered statistically significant.
Results
Clinical findings
Among 102 adult patients with ALK + ALCL, 18 (18%) cases had features of SC and/or LH variants and 84 cases (82%) had non-SC/LH patterns. The clinical features of these patients are summarized in Table 1. The SC/LH group included 8 men and 10 women with a median age of 35 years (range, 18–62 years) at time of diagnosis. Eleven of 16 (69%) patients had B-type symptoms. Lymphadenopathy was identified in 16 of 18 (89%) patients, and 9 of 17 (53%) had extra-nodal involvement. Bone marrow was involved in 4 of 16 (25%) patients and peripheral blood was involved in 3 of 5 (60%) patients examined (2 patients with SC variant, 1 patient with SC/LH pattern). Ten of 15 (67%) fully staged patients had stage III or IV disease. Three of 10 (30%) patients had leukocytosis (white blood cell count >11.0 × 109/L), and 1 of 9 (11%) patients had absolute lymphocytosis (lymphocyte count >4.8 × 109/L). Four of 10 (40%) patients tested showed an elevated serum lactate dehydrogenase level. Two of 13 patients (15%) had an International Prognostic Index (IPI) score of ≥3. The non-SC/LH group included 50 men and 34 women, with a median age of 33 years (range, 19–76 years).
Compared to patients with ALK + ALCL with non-SC/LH patterns, patients with SC/LH variants had more frequent peripheral blood involvement (60% vs. 0%, p = 0.02). There were no significant differences in other clinical features between patients with SC/LH versus non-SC/LH ALK + ALCL (all p > 0.05; Table 1).
Pathologic findings
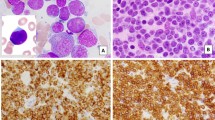
In SC variant, the neoplasm showed predominantly small to medium-size neoplastic cells with minimal cytoplasm, but some hallmark cells were usually present (Fig. 1). The neoplastic cells showed irregular or indented nuclei and often involved peripheral blood (Fig. 2). Bone marrow involvement was often subtle, with scattered and/or small clusters of small tumor cells observed (Fig. 2). The LH variant was characterized by: (1) abundant histiocytes in the background which can mask the neoplastic cells; and (2) neoplastic cells that are usually smaller than those in the common pattern, resembling the neoplastic cells in SC variant (Fig. 3).
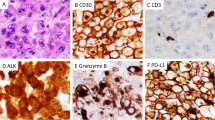
A At low magnification (×40), the architecture is relatively preserved, but sinusoids are expanded by a lymphoid infiltrate. B At high magnification (×400), the infiltrate is composed of small to medium-size lymphoma cells with irregular nuclei and scant cytoplasm (red arrows). A “hallmark” cell with a kidney-shaped nucleus is present (red arrowhead). The lymphoma cells are positive for CD30 (C; ×200), ALK (D, nuclear and cytoplasmic; ×200), CD3 (E; ×400), and granzyme B (F; ×200). Some lymphoma cells line up along blood vessels (C–F, red arrows). A–B Hematoxylin-eosin stain. C–F Immunohistochemistry.
A At low magnification (×40), the bone marrow biopsy does not show obvious lymphoma. B At high magnification (×400), small lymphoma cells are present (red arrows) that have round to irregular nuclei, open chromatin, and small distinct nucleoli. C The lymphoma cells are positive for ALK (nuclear and cytoplasmic, ×400). D Peripheral blood smears show small to medium-sized lymphoma cells with irregular or indented nuclei and scant cytoplasm (×1000). A, B Hematoxylin-eosin stain. C Immunohistochemistry. D Wright–Giemsa stain.
A At low magnification (×40), the nodal architecture is mostly effaced by a diffuse lymphohistiocytic infiltrate. B At high magnification (×400), the infiltrate is composed of scattered small to medium-size lymphoma cells (red arrows) in a background of abundant histiocytes. C CD68 highlights numerous histiocytes (×40). The lymphoma cells are positive for CD30 (D; ×40), ALK (E, ×40; insert, ×400, nuclear and cytoplasmic staining) and CD3 (F, weak, red arrows; ×400). The reactive T cells show strong CD3 positivity (F, black arrows). A, B Hematoxylin-eosin stain. C–F Immunohistochemistry.
The immunophenotypic features of these cases are summarized in Table 2. All tested cases of ALK + ALCL with features of SC/LH variants were positive for CD43 (n = 6) and 10 of 11 (91%) were positive for CD45. Most SC/LH neoplasms were positive for T-cell markers: CD2 (11/12; 92%), CD3 (13/16; 81%), CD4 (8/13; 62%), CD7 (8/10; 80%), CD8 (7/13; 54%), and CD25 (4/7; 57%). Four of 13 (31%) SC/LH cases were CD4/CD8 double positive (Table 2; Fig. 4). A small subset of SC/LH cases was positive for CD5 (5/14; 36%) and TCR alpha/beta (2/5; 40%). No cases (n = 5) were positive for TCR gamma/delta. Three of 6 (50%) ALK + ALCL cases with features of SC/LH variants were positive CD56. Figures 4 and 5 showed flow cytometric immunophenotyping plots of two cases. All tested SC/LH cases were positive for granzyme B (n = 8) and 5 of 6 (83%) cases were positive for EMA.
The lymphoma cells (pink dots) show increased side scatter (SSC) (A), and are positive for CD45 (A), CD4 (B, decreased), CD8 (B), CD3 (C, small subset), CD7 (C), CD2 (D, increased), and CD56 (F, partial), and are negative for CD5 (D), TCR alpha beta (E), and TCR gamma delta (E). The blue dots represent background reactive T cells.
The lymphoma cells (pink dots) show increased side scatter (SSC) (A) and are positive for CD45 (A), CD3 (B, partial), CD7 (B), CD8 (C), and CD2 (D, increased). The lymphoma cells are negative for CD4 (C), CD5 (D), TCR alpha/beta (E), TCR gamma/delta (E), and CD56 (F). The blue dots represent background reactive T cells.
Compared with ALK + ALCL cases with non-SC/LH patterns, cases of SC/LH variants were more often positive for CD2 (92% vs. 36%, p = 0.0007), CD3 (81% vs. 15%, p = 0.0001), CD7 (80% vs. 37%, p = 0.03), CD8 (54% vs. 7%, p = 0.0006). SC/LH cases also showed higher frequency of CD4/CD8 double positivity than non-SC/LH cases (31% vs. 3%, p = 0.0009). There were no significant differences in the expression of CD4, CD5, CD25, CD43, CD45, CD56, TCR alpha/beta, TCR gamma/delta, granzyme B, or EMA (all p > 0.05; Table 2).
Treatment and response
All patients were treated with various chemotherapy regimens over the time interval of this study, with or without stem cell transplant (SCT). In patients with SC/LH ALK + ALCL, 13 of 16 (81%) were treated with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or modified CHOP (Table 1). After initial induction chemotherapy, 11 of 15 (73%) patients achieved complete remission. Seven of 15 (47%) patients received stem cell transplant (SCT): 4 autologous and 3 allogeneic. In patients with non-SC/LH ALK + ALCL, 50 of 64 (78%) were treated with CHOP or a modified CHOP regimen and 50 of 62 (81%) achieved complete remission. Twenty of 57 (35%) were treated with SCT: 17 autologous, 2 allogeneic, and 1 autologous followed by allogeneic. There were no differences in initial treatment or complete remission rate between patients with ALK + ALCL with features of SC/LH variants versus non-SC/LH variants (all p > 0.05; Table 1).
Outcome
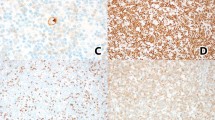
After a median follow-up of 30.8 months (range, 0.3–208 months), 19 of 87 (22%) patients died. Death occurred in 5 of 17 (29%) patients with ALK + ALCL with SC/LH patterns and in 17 of 72 (24%) patients ALK + ALCL with non-SC/LH patterns. High (>3) IPI scores were associated with shorter OS in adults with ALK + ALCL (p = 0.003; Fig. 6A), although there was no significant difference in PFS between patients with high versus low IPI scores (p = 0.88; Fig. 6B). No significant differences were observed in overall or PFS between patients with ALK + ALCL with SC/LH patterns versus non-SC/LH patterns (p = 0.99 and 0.94, respectively; Fig. 6C, D).
Discussion
ALK + ALCL is most common in children and young adults and about one-third of all cases reported in children have a SC or LH pattern. One study of 13 ALCL patients (7 adults and 6 children) with LH variant reported that these neoplasms occurred in young patients with a mean age of 14.8 years9. In two cohorts of pediatric patients with ALK + ALCL, the SC and LH variants were present in 29% (24/82) and 35% (26/75) of ALK + ALCL cases, respectively21,22. A large study of 361 pediatric ALK + ALCL patients showed that 32% of cases had a component of SC or LH variant15. There are very limited studies of adults with ALK + ALCL with a SC and/or LH variant in the literature. In one study, 8 of 43 (19%) cases of ALK + ALCL in adults had SC/LH patterns11. In the present study, SC and/or LH variants were present in 18% of adult ALK + ALCL cases, suggesting a lower frequency of SC/LH morphology in adults than in children with ALK + ALCL.
The SC and LH variants of ALK + ALCL can pose a challenge for establishing the correct diagnosis. The small neoplastic cells in the SC variant usually show CD30 expression by only a subset of cells, often less than 5–10%, which can result in an incorrect diagnosis of reactive/inflammatory process (when normal architecture is relatively preserved) or peripheral T-cell lymphoma, NOS (when normal architecture is effaced). The large number of histiocytes present in the LH pattern may mask the neoplastic cells which are often only a minor component of the infiltrate, also leading to misdiagnosis of reactive process9,10,14,23. Immunohistochemical studies for CD30 and ALK are essential for establishing the correct diagnosis. Although the CD30+ tumor cells in ALK + ALCL with SC/LH patterns are small, mimicking CD30+ immunoblasts seen in reactive lymph nodes, the location of CD30+ cells can be useful for distinguishing between reactive processes and ALK + ALCL. In benign lymph nodes, CD30+ immunoblasts are usually located within or near the edge of the B cell follicles. In contrast, CD30+ tumor cells in ALK + ALCL with SC/LH patterns often cluster around blood vessels or within sinuses.
Most ALK + ALCL cases lack one or more T-cell antigens; in particular, CD3 and CD5 are often negative5,6,24. Although loss of T-cell markers is common in ALCL in general, an early study of 123 ALK + ALCL cases (including children and adults) reported that a T-cell immunophenotype (based on CD3, CD43, CD45RO, and CBF78) was more often seen in cases with a SC, LH, or mixed SC/LH pattern than in cases with a common pattern11. In that study, the SC population was found to show stronger expression of T-cell markers as compared with the larger anaplastic cells. A “null” immunophenotype is rarely identified in ALK + ALCL with SC/LH patterns11. Similarly, a study of 361 childhood ALK + ALCL cases reported an association between higher CD3 positivity and a component of SC/LH variants15. In contrast, in a study of 128 pediatric patients with ALK + ALCL by Abramov et al., there was no significant difference in CD3, but cases with a non-common type pattern more frequently expressed CD8 and less often expressed CD5 than cases with a common pattern18. In the cohort of adults with ALK + ALCL that we report here, cases of SC/LH variants were associated with a higher frequency of being positive for CD2, CD3, CD7, and CD8, but not CD5.
Although B- and T- cell lymphomas can present with or evolve into a leukemic phase of disease, <5% of patients with ALK + ALCL have a leukemic phase25. Most cases of ALK + ALCL in leukemic phase reported have been SC variant, and most patients were younger than 30 years of age25,26,27,28,29,30. In this study cohort, adults with ALK + ALCL with SC/LH patterns (all having a SC component) more often had peripheral blood involvement than patients with non-SC/LH patterns, confirming the reported association between SC morphology and leukemic phase.
Patients with ALK + ALCL usually have a good prognosis with 5-year survival rates of 70–90%3,4,5,6,8. Most studies in the literature have shown an association between SC/LH variants and poorer clinical outcomes in pediatric ALK + ALCL patients, although one early study of 82 children with ALK + ALCL reported that histologic variant was not a prognostic factor21. In a study of 9 patients (children and adults) with the SC variant of ALCL, the 2-year disease-free survival was 14% and the OS was 51%12. Similarly, a study of 17 patients with LH variant ALCL reported that the survival probability after 40 months was only 47%16. In a study of 80 children with ALK + ALCL, non-common patterns were a poor prognostic factor in multivariate analysis17. A more recent study of 361 childhood ALK + ALCL cases by Lamant et al. reported that the presence of a component of SC/LH variants was associated with treatment failure, indicating that SC/LH pattern is an adverse prognostic factor15. Their findings were confirmed by a subsequent study in which a non-common pattern was associated with poorer event-free and OS in pediatric patients with ALK + ALCL18. The mechanisms underlying the poorer prognosis of the SC/LH variants in children with ALK + ALCL are unknown. High anti-ALK antibody titers were detected more often in ALK + ALCL patients with a common pattern, as opposed to patients with non-common histologic patterns; increasing antibody titers were also associated with a decreased risk of relapse31. Therefore, it has been speculated that there may be host immune deficiency against the neoplastic cells in patients with SC/LH ALK + ALCL. In addition, SC/LH variants in children have been associated with a positive NPM-ALK result detected by reverse transcription (RT)-PCR and real-time quantitative (RQ)-PCR in bone marrow at diagnosis and increased frequency of treatment failure15,17. In contrast, the results of the present study in adults with ALK + ALCL show no association between the SC/LH variants and survival.
In conclusion, we assessed morphologic variants (SC/LH versus non-SC/LH) in 102 adults with ALK + ALCL and compared the clinicopathologic features and outcome between these two groups. We found that the presence of a SC and/or LH pattern is associated with peripheral blood involvement, CD8 + positivity and retention of expression of the T markers CD2, CD3, and CD7. In addition, in contrast with children with ALK + ALCL, in this study of adults with ALK + ALCL, the presence of SC and/or LH variants has no impact on prognosis.
Data availability
All data has been presented in the manuscript, Tables and Figures.
References
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 127, 2375–2390 (2016).
Medeiros, L. J. & Elenitoba-Johnson, K. S. Anaplastic large cell lymphoma. Am J Clin Pathol 127, 707–722 (2007).
Vose, J., Armitage, J., Weisenburger, D. & International, T. C. L. P. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J. Clin. Oncol. 26, 4124–4130 (2008).
Falini, B. et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood 93, 2697–2706 (1999).
Sibon, D. et al. Long-term outcome of adults with systemic anaplastic large-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte trials. J. Clin. Oncol. 30, 3939–3946 (2012).
ten Berge, R. L. et al. ALK-negative anaplastic large-cell lymphoma demonstrates similar poor prognosis to peripheral T-cell lymphoma, unspecified. Histopathology 43, 462–469 (2003).
Parrilla Castellar, E. R. et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 124, 1473–1480 (2014).
Ellin, F., Landstrom, J., Jerkeman, M. & Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood 124, 1570–1577 (2014).
Pileri, S. et al. Lymphohistiocytic T-cell lymphoma (anaplastic large cell lymphoma CD30+/Ki-1 + with a high content of reactive histiocytes). Histopathology 16, 383–391 (1990).
Summers, T. A. & Moncur, J. T. The small cell variant of anaplastic large cell lymphoma. Arch Pathol. Lab. Med. 134, 1706–1710 (2010).
Benharroch, D. et al. ALK-positive lymphoma: a single disease with a broad spectrum of morphology. Blood 91, 2076–2084 (1998).
Kinney, M. C. et al. A small-cell-predominant variant of primary Ki-1 (CD30)+ T-cell lymphoma. Am. J. Surgical Pathol. 17, 859–868 (1993).
Stein, H. et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 96, 3681–3695 (2000).
Tsuyama, N., Sakamoto, K., Sakata, S., Dobashi, A. & Takeuchi, K. Anaplastic large cell lymphoma: pathology, genetics, and clinical aspects. J. Clin. Exp. Hematop. 57, 120–142 (2017).
Lamant, L. et al. Prognostic impact of morphologic and phenotypic features of childhood ALK-positive anaplastic large-cell lymphoma: results of the ALCL99 study. J. Clin. Oncol. 29, 4669–4676 (2011).
Klapper, W., Bohm, M., Siebert, R. & Lennert, K. Morphological variability of lymphohistiocytic variant of anaplastic large cell lymphoma (former lymphohistiocytic lymphoma according to the Kiel classification). Virchows Arch. 452, 599–605 (2008).
Damm-Welk, C. et al. Prognostic significance of circulating tumor cells in bone marrow or peripheral blood as detected by qualitative and quantitative PCR in pediatric NPM-ALK-positive anaplastic large-cell lymphoma. Blood 110, 670–677 (2007).
Abramov, D. et al. Expression of CD8 is associated with non-common type morphology and outcome in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma. Haematologica 98, 1547–1553 (2013).
Lyapichev, K. A. et al. MYC expression is associated with older age, common morphology, increased MYC copy number, and poorer prognosis in patients with ALK+ anaplastic large cell lymphoma. Hum. Pathol. 108, 22–31 (2021).
Shen, J. et al. CD8 expression in anaplastic large cell lymphoma correlates with noncommon morphologic variants and T-cell antigen expression suggesting biological differences with CD8-negative anaplastic large cell lymphoma. Hum. Pathol. 98, 1–9 (2020).
Brugieres, L. et al. CD30(+) anaplastic large-cell lymphoma in children: analysis of 82 patients enrolled in two consecutive studies of the French Society of Pediatric Oncology. Blood 92, 3591–3598 (1998).
d’Amore, E. S. et al. Anaplastic large cell lymphomas: a study of 75 pediatric patients. Pediatr. Dev. Pathol. 10, 181–191 (2007).
Leventaki, V., Bhattacharyya, S. & Lim, M. S. Pathology and genetics of anaplastic large cell lymphoma. Semin. Diagn Pathol. 37, 57–71 (2020).
Savage, K. J. et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood 111, 5496–5504 (2008).
Spiegel, A. et al. Paediatric anaplastic large cell lymphoma with leukaemic presentation in children: a report of nine French cases. Br. J. Haematol. 165, 545–551 (2014).
Nguyen, J. T. et al. Anaplastic large cell lymphoma in leukemic phase: extraordinarily high white blood cell count. Pathol. Int. 59, 345–353 (2009).
Onciu, M. et al. ALK-positive anaplastic large cell lymphoma with leukemic peripheral blood involvement is a clinicopathologic entity with an unfavorable prognosis. Report of three cases and review of the literature. Am. J. Clin. Pathol. 120, 617–625 (2003).
Ok, C. Y., Wang, S. A. & Amin, H. M. Leukemic phase of ALK(+) anaplastic large-cell lymphoma, small-cell variant: clinicopathologic pitfalls of a rare entity. Clin. Lymphoma Myeloma Leuk. 14, e123–e126 (2014).
Bayle, C. et al. Leukaemic presentation of small cell variant anaplastic large cell lymphoma: report of four cases. Br. J. Haematol. 104, 680–688 (1999).
Grewal, J. S. et al. Highly aggressive ALK-positive anaplastic large cell lymphoma with a leukemic phase and multi-organ involvement: a report of three cases and a review of the literature. Ann. Hematol. 86, 499–508 (2007).
Ait-Tahar, K. et al. Correlation of the autoantibody response to the ALK oncoantigen in pediatric anaplastic lymphoma kinase-positive anaplastic large cell lymphoma with tumor dissemination and relapse risk. Blood 115, 3314–3319 (2010).
Funding
The study was partly supported by The University of Texas MD Anderson Cancer Center Division of Pathology and Laboratory Medicine Research Grant.
Author information
Authors and Affiliations
Contributions
M.K. collected data and wrote the manuscript; S.L. and L.J.M. designed the study and wrote the manuscript; R.N.M., S.I., C.C.Y. and G.T. collected data and wrote the manuscript; S.K., P.L. and F.V. wrote the manuscript; L.Q. collected data; J.X. designed the study, collected data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical Approval and Consent to Participate
The study was approved by the institutional review board at The University of Texas MD Anderson Cancer Center.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khanlari, M., Li, S., Miranda, R.N. et al. Small cell/lymphohistiocytic morphology is associated with peripheral blood involvement, CD8 positivity and retained T-cell antigens, but not outcome in adults with ALK+ anaplastic large cell lymphoma. Mod Pathol 35, 412–418 (2022). https://doi.org/10.1038/s41379-021-00944-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00944-1
This article is cited by
-
Anaplastic large cell lymphoma, ALK-negative exhibiting rare CD4 [ +] CD8 [ +] double-positive immunophenotype
Journal of Hematopathology (2022)