Abstract
A novel 3-tiered grading system based on tumor budding activity and cell nest size has been validated to be highly prognostic in organ-wide squamous cell carcinomas. In this study, we applied a similar grading system with slight modification to assess the prognostic value in an institutional cohort of well annotated endocervical adenocarcinomas (EAC) consisting of 398 consecutive cases with surgical resection, no neoadjuvant chemotherapy, and higher than stage pT1a. Each case was reviewed by the International Endocervical Adenocarcinoma Criteria and Classification (IECC) and Silva pattern classification, and scored on tumor budding activity and cell cluster size to form the basis of a novel grading system. High budding activity, small tumor cell cluster size, and novel grade 3 were more frequently associated with a decreased overall survival time and tumor recurrence time (p < 0.001), and several other clinicopathologic factors including HPV-independent adenocarcinoma, lymphovascular invasion, lymph node metastasis, advanced FIGO stage, and Silva pattern C (p < 0.05). Moreover, the novel grading system was helpful in stratifying overall survival in HPV-associated adenocarcinoma (p = 0.036) and gastric-type adenocarcinoma (p = 0.033). On multivariate analysis, novel grade 3 was an adverse indicator for overall survival and tumor recurrence independently of age and FIGO stage (p < 0.05). By comparison, Silva pattern C was only associated with tumor relapse (p = 0.020) in HPV-associated adenocarcinomas whereas the conventional FIGO system was not associated with overall survival and tumor recurrence in EAC (p > 0.05). In conclusion, our study demonstrates that the grading system based on tumor budding activity and cell cluster size is robust in prognostic assessment that outperforms the conventional FIGO grading and Silva pattern classification in EAC. The novel grading system, if further validated, could be applicable in routine pathologic descriptions of EAC by providing useful information in clinical decision-making.
Similar content being viewed by others
Introduction
Cervical carcinoma remains the fourth leading cancer in women worldwide.1 Endocervical adenocarcinoma (EAC) is the second most common histotype following squamous cell carcinoma (SCC), accounting for 10–25% of all cervical carcinomas. EAC usually has a poorer prognosis than SCC despite the same treatment strategies in both cancers.2 EAC shows a highly variable disease course, not only ranging from early stage to advanced stage with wide dissemination and metastasis, but it is also a heterogeneous group in terms of its subtypes. Valid prognosticators are critically required for tailored treatment of EAC.
There have been significant advances in our understanding of the prognostic assessment of EAC in recent years, such as revised tumor classification and pattern-based systems.3,4 The International Endocervical Adenocarcinoma Criteria and Classification (IECC) scheme classified EAC into HPV-associated adenocarcinoma (HPVA) and HPV-independent adenocarcinoma (HPVI), based on etiology (hrHPV infection) and related surrogate morphology.3 The IECC approach appears to be valuable for prognostic assessment in EAC5; therefore, it has been adopted by the recently published fifth edition of the World Health Organization (WHO) classification of female genital tumors.6 The Silva Pattern Classification subdivides HPVA into 3 prognostic groups, correlating the presence and extent of destructive stromal invasion, nuclear grade and structure with lymph node metastasis and clinical outcomes.4
Several pathological parameters are helpful in the assessment of prognosis in cervical cancers, such as histotype, tumor size, depth of invasion, and lymphovascular invasion (LVI).7,8 Tumor grade is a conventional prognostic factor in many cancers. However, in EAC, tumor grading, including grading criteria and its clinical significance, has received scant coverage in the literature. Recently, the International Society of Gynecological Pathologists (ISGyP) suggested the revised International Federation of Gynecology and Obstetrics (FIGO) system for uterine endometrioid carcinoma for EAC grading based on the available evidence and its common application in clinical settings.9 There is no consensus on the grading criteria and clinical significance in EAC; therefore, the issue remains unresolved to date. On account of an expectation from clinicians, tumor grading remains as a recommended (non-core) but not a required (core) element in the recent International Collaboration on Cancer Reporting data set for reporting cervical SCC and EAC.10 It is an important requirement to develop a novel grading system with prognostic value and potential universal application.
Tumor budding, defined as isolated cells or small tumor cell clusters consisting of <5 neoplastic cells that “bud” into the peritumoral stroma, conceptually represents a common form of destructive stromal invasion.11,12 Data have accumulated to indicate that increased tumor budding is a strong adverse prognostic factor in many cancers.12,13,14 Based on the combination of tumor budding activity and cell nest size, a second parameter that qualitatively measures the capability of cancer cell dissociation, a novel grading approach with substantial clinical significance has been established for SCC from several anatomic sites including uterine cervix.15,16,17,18,19,20 High tumor budding count has been found to be associated with decreased disease-free and cancer-specific survival in patients with early-stage EAC recently.21 Nevertheless, the clinical value of cell nest size (the term, “cluster” is preferred in EAC) and the novel grading approach has not been investigated in EAC yet.
In this study, we compared the clinical significance between the novel grading approach based on tumor budding and cell cluster size and the revised FIGO grading system in a large cohort of EAC patients from a major Chinese university women’s hospital. We also analyzed the relationship between histological grading and other histopathologic and molecular parameters, such as IECC histotypes, Silva pattern, and p53 staining. Our specific aim is to evaluate its applicability and prognostic performance in EAC.
Materials and methods
Case selection
Consecutive cases of cervical adenocarcinomas in a resected specimen were retrieved from the archives of the Department of Surgical Pathology, Women’s Hospital, School of Medicine, Zhejiang University, PR China, between January 2004 and December 2019. We excluded patients with biopsy only, neoadjuvant chemotherapy, pT1a1 EAC, and the serous type and the endometrioid type if they had concurrent carcinomas in the upper female genital tract. The clinical data were extracted from the electronic medical records before de-identification. Tumor stage was re-assessed according to the 2018 FIGO cervical cancer staging system.22 Histologic slides and follow-up data were available for review. The hospital’s Institutional Review Board approved this study (IRB: 20170139).
Histologic assessment
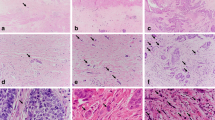
Two authors (SH and YL), who were blinded to the clinical data and follow-up, independently reviewed the archival H&E slides for detailed morphological analysis. Cases resulting in interpretational disagreement between the two authors were submitted to the third pathologist (LB). All tumors were graded by evaluating the additive scores of tumor budding and cell nest size [Table 1]. A similar approach with slight modification has been reported in SCC from various organs previously.15,16,17,18,19,20 Briefly, tumor budding activity was recorded in the tumor area having the highest budding activity (hotspot) after assessment throughout the whole tumor area. Tumor budding activity was scored as 1, 2 and 3 for 0, 1–14 (low tumor budding activity) or ≥15 budding foci (high tumor budding activity) in 10 high power fields (HPFs) with a total area of 2.37 mm2 (Leica DMR 2000; the field diameter of a 10× objective: 22 mm), respectively. Cell cluster size was evaluated according to the minimal size of invasive tumor cell nests. Cell cluster size comprising 2–4 (small), 5–15 (intermediate), and >15 tumor cells (large) had scores of 3, 2 and 1, while single tumor cells detached within the stroma received a score of 4. Representative images are illustrated in Fig. 1. The number of tumor cell clusters with minimal size did not influence the cell cluster size scoring. Tumors with pure glands (no tumor cell clusters or single cells) were frequently present in EAC. Cell cluster size score 1 was designated for these tumors given that they were well differentiated by conventional grading whereas cell clusters lacking glandular formation were believed to be poorly differentiated.23 The sum of tumor budding and cell cluster size scores generated a final grading score ranging from 2 to 7. A grading score of 2–3, 4–5, and 6–7 were defined as grade 1 (G1, well differentiated), 2 (G2, moderately differentiated), and 3 (G3, poorly differentiated), respectively. This grading approach based on tumor budding will be referred to as “novel grade” in the following sections of the manuscript.
Tumor budding (A–C): overview (A × 50) and details (B × 200) of an EAC with high tumor budding activity, indicated by the branching of numerous small tumor clusters of <5 cells into the surrounding tissue; details of low tumor budding activity with 2 clusters of tumor cells, each showing budding (arrows) into the tumor stroma (C × 200). Cell nest size (D–F): single cell invasion (D × 200), intermediate (E × 200) and large (F × 200) sized cell clusters.
The tumors were reclassified according to the IECC.3 The Silva pattern classification was applied in HPVA.4 All cases were also graded in accordance with the revised FIGO system recommended from ISGyP.9 Tumors with ≤10%, 11–50%, and >50% solid growth were designated as grade 1, 2, and 3, respectively. Tumors were upgraded if marked nuclear atypia was present in the majority (>50%) of the tumor cells. The FIGO grading was not recommended for HPVI, particularly gastric-type adenocarcinoma (GAC), because of their aggressive clinical behavior even in cases with deceptively bland (low grade) histology.9,24 Nevertheless, in this study, we retained FIGO grading in GAC for comparison with the novel grading scheme.
Immunohistochemistry
Immunohistochemistry (IHC) was carried out on 4-µm thick sections from neutral-buffered formalin-fixed, paraffin-embedded tissue blocks. The antibodies included p16 (G175-405; BD Bioscience, San Jose, CA; 1:100), MUC6 (MRQ-20; Cell Marque, Rocklin, USA; ready-to-use), and p53 (DO-7; Thermo Fisher Scientific, Waltham, USA; 1:300). An EnVision immunostaining procedure (DAKO, Carpentaria, USA) was performed according to the manufacturer’s protocols. Strong, diffuse nuclear staining (>80% cells with deep staining) in EAC and null expression in HPVI were defined as aberrant p53 expression; otherwise, as wild-type expression. Two patterns of p16 immunoreactivity were identified – widespread, diffuse staining (block staining) and patchy positivity. Block p16 staining and positive MUC6 staining (>10% positive cells) was applied to aid the diagnosis of HPVA and GAC, respectively.
Statistics
Statistical analysis was performed using the SPSS 16.0 software package (SPSS, Inc., Chicago, IL, USA). Non-parametric tests (Wilcoxon test or Mann–Whitney U test), χ2 test, and one-way analysis of variance were used to determine the clinicopathologic significance among tumors with different histologic grade. The Kaplan–Meier method was performed to compare mortality and recurrence between groups, and the log-rank test was used to analyze the statistical significance of the differences between groups. Multivariate survival analysis was carried out by the Cox’s proportional regression hazard (HR) method. We adjusted multiple parameters that were found to be significant in the univariate model. The statistical threshold was set at 0.05 (two-sided).
Results
General clinicopathologic features
The study cohort had 398 EAC cases after excluding adenosquamous carcinoma, endometrioid and serous carcinoma from the upper genital tract. The age of the patients ranged from 19 to 77 years (median 46 years, mean 46.6 ± 9.9 years). Most patients (n = 356) underwent radical abdominal hysterectomy with bilateral salpingo-oophorectomy and pelvic lymph-node dissection. Thirty-three patients were at FIGO stage IA, 274 at stage IB, 32 at stage II, 48 at stage III, 7 at stage IV and 4 unstaged. The patients were followed up for 3–185 months (median time: 26 months). Thirty-one patients recurred with a median time of 24 months (range: 1–142 months) post-operatively. Twenty patients died of disease (median survival time: 17 months; range: 3–144 months).
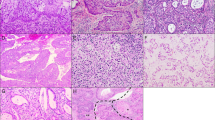
The IECC types included 294 HPVA (73.8%), 88 HPVI (22.1%) and 16 adenocarcinoma-not otherwise specified (4.1%). Usual-type EAC (224/294, 76.2%) was the main histotype in HPVA while GAC (73/88, 83.0%) was predominant in HPVI. The other HPVI subtypes included clear cell carcinoma (n = 10), endometrioid carcinoma (n = 4) and mesonephric adenocarcinoma (n = 1). In HPVA, the Silva pattern included pattern A in 58 patients (19.7%), pattern B in 46 cases (15.6%) and pattern C in 190 cases (64.6%). LVI and nerve involvement (NI) were present in 165 (41.5%) and 29 (7.3%) cases, respectively. Aberrant p53 expression was observed in 48 of 357 (13.4%) cases. Representative morphology and IHC are shown in Fig. 2A–D.
Distribution of tumor budding, minimal cell cluster size and novel grading
The distribution of tumor budding activity included 264 (66.3%) cases without any activity, 52 (13.1%) cases with low activity (<15/10HPF) and 82 (20.6%) with high activity (≥15/10HPF). The assessment of minimal tumor cell cluster size indicated that large clusters (>15 cells or pure glands) were present in 217 (54.5%) cases, intermediate clusters (5–15 cells) in 47 (11.8%), small clusters (2–4 cells) in 48 (12.1%), and single cell invasion in 86 (21.6%) tumors. High budding activity was positively correlated with small cell cluster size (r = 0.984, p < 0.001). The novel grade based on the combination of tumor budding activity and tumor cell cluster size scores identified that 264 (66.3%) cases were well differentiated (G1), 28 (7.0%) moderately differentiated (G2), and 106 (26.7%) poorly differentiated (G3).
Correlation of tumor budding, minimal cell cluster size and novel grade with clinicopathologic parameters and prognosis
The correlation of tumor budding, minimal cell cluster size and novel grade with clinicopathologic parameters is summarized in Table 2. Briefly, high budding activity, small tumor cell cluster size and single cells, plus novel grade 3 were more frequently associated with HPVI, lymph node metastasis, ovarian involvement, LVI, PNI, invasion of deep cervical wall, advanced FIGO stage, and aberrant p53 expression in EAC as well as Silva pattern C in HPVA (p < 0.05).
Univariate survival analysis indicated that tumor budding, cell cluster size and novel grade were significantly associated with overall survival and tumor recurrence in EAC (all p < 0.001, Table 3). High budding activity, small cluster size and single cells, and high novel tumor grade showed a decreased overall survival time and tumor recurrence time (Fig. 3A–F). Moreover, novel grade 3 was an adverse indicator for overall survival in HPVA (p = 0.036) and GAC (p = 0.033) (Fig. 3G, H) as well as early tumor recurrence in HPVA (p = 0.004) while Silva pattern C was only associated with tumor relapse (p = 0.020) in HPVA. Multivariate analysis demonstrated that the novel grading system was significantly associated with overall survival and tumor recurrence independently of age and FIGO stage in EAC patients (p < 0.05, Table 4).
Correlation of revised FIGO grading system with clinicopathologic parameters and prognosis
By revised FIGO grading,9 73.1% (n = 291) of EAC cases were well differentiated (G1), 14.8% (n = 59) were moderately differentiated (G2), and 12.1% (n = 48) were poorly differentiated (G3). The revised FIGO system did not significantly correlate with the novel grading system (p = 0.398, r = −0.057). Among the clinicopathologic parameters with potential prognostic value, the revised FIGO G3 was the only one positively associated with LVI and deep invasion of the cervical wall in EAC, and Silva pattern C in HPVA (p < 0.05, Table 2). Survival analysis indicated that the revised FIGO system was not associated with overall survival or tumor recurrence in EAC (p > 0.05, Table 3).
Discussion
The histopathologic grading system based on tumor budding and cell nest size has been validated as prognostically useful in patients with SCC from several anatomic sites, including lung,15 esophagus,16 larynx/hypopharynx,18 and oral cavity.17 Two recent studies on uterine cervical SCC have indicated that the novel grading system can provide more powerful prognostic information than the WHO-based grading system.19,20 These studies have unanimously concluded that the combination of tumor budding activity and cell nest size can stratify organ-wide SCC patients into subgroups with good, intermediate and poor prognosis. Moreover, the grading system had minimal methodological variations across studies, a high concordance among pathologists, and easy applicability in clinical practice. The grading approach based on tumor budding has demonstrated considerable clinical utility in prognostic assessment, and may potentially contribute to future decision-making in SCC from many anatomic sites.
In this study, we investigated the prognostic significance of the novel grading scheme incorporating tumor budding and tumor cluster size from a large cohort of resected EAC. By analogy with SCC,15,16,17,18,19,20 we confirmed that tumor budding activity and cell cluster size were powerful histology-based prognostic factors, and the combination of both parameters resulted in an easily applicable, 3-tiered grading system of major prognostic significance. The grading system is also helpful to stratify HPVA and GAC patients in terms of overall survival. Notably, high tumor budding activity, small cell cluster size and novel grade 3 were additionally closely associated with several important prognostic variables, such as HPVI, lymph node metastasis, LVI, invasion of the deep cervical wall, advanced FIGO stage, aberrant p53 expression and Silva pattern C.3,4,5,7,8,25 The biological basis for the prognostic performance of the novel grading system is not understood, but the prevailing hypothesis is that tumor budding and cell clusters may be associated with dissociation, plasticity, motility, invasiveness and epithelial-mesenchymal transition in cancer cells.11,12,26 The underlying molecular events may be linked to RAS oncogenic mutations, nuclear ß-catenin and reduced E-cadherin expression.26 In our study, we have found that increased tumor budding and minimal cell clusters correlated with mutant-type p53 expression, which may cause severe dysregulation of cellular growth and result in poor prognosis in cancers.27
While the effects of the novel grading system have not been investigated in adenocarcinomas yet, high tumor budding activity or score has been well established as an indicator of poor prognosis in adenocarcinomas from several anatomic locations, such as colorectum,13,28 small intestine,29 esophagus,30 stomach,14 pancreas,31 breast,32 liver,33 lung,34 uterine corpus35 and cervix.21 Recently, a large colorectal cancer cohort comprising 1004 cases has shown that histologic subtypes and tumor budding, but not WHO grade, are stage-independent prognosticators for patient survival; therefore, tumor budding is superior to WHO grade in prognostic prediction in colorectal cancers.28 Satabongkoch et al.21 found that usual-type EAC patients with a high tumor bud count had a significant decrease in both disease-free survival and cancer-specific survival on univariate analysis, but did not on multivariate analysis. Poorly differentiated clusters, defined as solid cancer cell nests comprising five or more cancer cells and lacking glandular formation, have been validated to be a highly reproducible and relevant factor for predicting the prognosis and metastatic risk in patients with colorectal cancers,23 and may provide more robust prognostic information than tumor budding and conventional grading.36,37 In EAC, the prognostic value of tumor cluster size has not been specifically investigated yet. In our study, we slightly adjusted the algorithm of cell nest size proposed for SCC by designating cell cluster size score 1 for EAC with pure glandular formation (no cell clusters or detached single cells) in contrast with the poor differentiation represented by cell clusters lacking glandular formation.23,29,36,37 We found that small cell cluster was a significant indicator for poor overall survival and tumor recurrence in EAC. Despite the presence of strong correlation between tumor budding activity and tumor cell cluster size, we believe that the combination of both parameters can more precisely reflect the malignant potential of EAC than a single factor, generating a highly prognostic grading system in EAC. Likewise, Jesinghaus et al.19 suggested that the two factors may assess the ability for cellular dissociation in cancers from different angles: cell nest size qualitatively describing the degree of cellular discohesion, and tumor budding being a quantitative parameter to measure the amount of dissociative growth within a given cancer.
Conventional histologic grading of EAC according to the revised FIGO system of uterine endometrioid carcinoma uses the combination of solid architecture and cytologic features.9 A few studies have evaluated the clinical outcomes against conventional histologic grading in EAC. Baalbergen et al.38 found that FIGO stage, grade and lymph node metastasis were significant independent predictors of 5-year survival in 305 cervical adenocarcinomas of mixed types. Another study on 129 HPVA indicated that grade 3 histology was the only independent predictor of decreased disease-free and cancer-specific survival on multivariate analysis.21 In contrast, other studies did not find that histologic grade was an independent prognostic factor.5,39 GAC, the predominant histotype of HPVI, is a clinically aggressive tumor with poor prognosis, irrespectively of degree of differentiation.24 In accordance with a recent study using IECC criteria in 205 EAC,5 our study indicated that conventional histologic grade was not statistically significant with regard to prognosis in HPVA, HPVI, or both. The findings support the concept that conventional tumor grading is a questionable major prognostic factor in EAC and has limited influence on treatment algorithms guiding patient management.9
The Silva pattern classification is based on pattern of growth and invasion, and takes into account tumor architecture and nuclear grade. It is capable of stratifying HPVA into subgroups with no (pattern A), minimal (pattern B) and high risk (pattern C) of lymph node metastasis.4 Importantly, the reproducibility and prognostic value of the Silva and IECC classifications have been well documented.25 However, the Silva pattern system is suitable only for HPVA rather than HPVI. A combination of Silva pattern classification and IECC tumor type emerged to form the basis for prognostic stratification of EAC, with the Silva pattern acting as a surrogate for tumor grade: pattern A and B tumors being well to moderately differentiated and pattern C tumors having poor differentiation architecturally.9 In our study, we found that Silva pattern C was associated with tumor recurrence by univariate analysis, but was not an independent variable by multivariate analysis. By comparison, the novel grade was significantly associated with overall survival and tumor recurrence independently of age and FIGO stage. Moreover, it can stratify GAC, the major type of HPVI, into two subgroups with different prognosis –thereby, potentially contributing to decision-making in the treatment of this uncommon aggressive cancer. Collectively, in terms of prognostic assessment, the novel grade appears to outperform both the Silva pattern scheme and conventional histologic grade.
Our retrospective study is limited by relatively short follow-up time, a small number of rare subtypes, and a restriction to resected specimens that impacts on the transferability of this novel grading to biopsy specimens. Further multi-institutional investigation of a larger number of tumors, both resected and biopsy specimens, with longer follow-up time is critically required to consolidate the prognostic significance of the novel grading system in EAC and its subtypes. As in SCC, an organ-wide study may also expand the potential clinical application of the tumor grade scheme.
In conclusion, our retrospective study on a large case series has verified that a 3-tiered grading system based on tumor budding activity and cell cluster size is a prognostic indicator that appears to be superior to the conventional FIGO grading and Silva pattern classification in EAC. The novel grading system can be applicable in the prognostic stratification of various EAC subtypes including HPVA and GAC. Our findings validate previous studies that proposed tumor budding and poorly differentiated clusters for prognostic stratification in pancreatic and colorectal adenocarcinoms as well as the similar grading system for organ-wide SCC patients. If further confirmed, the novel grading system would be applied in pathologic descriptions of EAC routinely, with the potential to enhance prognostic accuracy and influence treatment algorithms in EAC patients in the future.
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
Fujiwara, K., Monk, B. & Devouassoux-Shisheboran, M. Adenocarcinoma of the uterine cervix: why is it different? Curr. Oncol. Rep. 16, 416 (2014).
Stolnicu, S. et al. International Endocervical Adenocarcinoma Criteria and Classification (IECC): a new pathogenetic classification for invasive adenocarcinomas of the endocervix. Am. J. Surg. Pathol. 42, 214–226 (2018).
Roma, A. A. et al. Invasive endocervical adenocarcinoma: a new pattern-based classification system with important clinical significance. Am. J. Surg. Pathol. 2015, 667–672 (2015).
Stolnicu, S. et al. Clinical outcomes of HPV-associated and unassociated endocervical adenocarcinomas categorized by the International Endocervical Adenocarcinoma Criteria and Classification (IECC). Am. J. Surg. Pathol. 43, 466–474 (2019).
Herrington, C. S. et al. Chapter 8: Tumours of the uterine cervix. In: The WHO Classification of Tumors Editorial Board, editors. WHO Classification of Tumors of Female Reproductive Organs. 5th ed. Lyon, France: International Agency for Research on Cancer (IARC), 2020. p. 336–389.
Halle, M. K. et al. Clinicopathologic and molecular markers in cervical carcinoma: a prospective cohort study. Am. J. Obstet. Gynecol. 17, 432.e1–432.e17 (2017).
Takeda, N. et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet. Gynecol. Scand. 81, 1144–1151 (2002).
Talia, K. L. et al. Grading of endocervical adenocarcinomas: review of the literature and recommendations from the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 40, S66–S74 (2021).
McCluggage, W. G. et al. Data set for the reporting of carcinomas of the cervix: recommendations from the International Collaboration on Cancer Reporting (ICCR). Int. J. Gynecol. Pathol. 37, 205–228 (2017).
Smedt, L. et al. Expression profiling of budding cells in colorectal cancer reveals an EMT-like phenotype and molecular subtype switching. Br. J. Cancer 116, 58–65 (2016).
Hong, K. O. et al. Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process. Hum. Pathol. 80, 123–129 (2018).
Lugli, A. et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 30, 1299–1311 (2017).
Kemi, N., Eskuri, M., Ikäläinen, J., Karttunen, T. J. & Kauppila, J. H. Tumor budding and prognosis in gastric adenocarcinoma. Am. J. Surg. Pathol. 43, 229–234 (2019).
Weichert, W. et al. Proposal of a prognostically relevant grading scheme for pulmonary squamous cell carcinoma. Eur. Respir. J. 47, 938–946 (2016).
Jesinghaus, M. et al. A novel grading system based on tumor budding and cell nest size is a strong predictor of patient outcome in esophageal squamous cell carcinoma. Am. J. Surg. Pathol. 41, 1112–1120 (2017).
Boxberg, M. et al. Tumor budding activity and cell nest size determine patient outcome in oral squamous cell carcinoma: proposal for an adjusted grading system. Histopathology 70, 1125–1137 (2017).
Boxberg, M. et al. Tumor budding and cell nest size are highly prognostic in laryngeal and hypopharyngeal squamous cell carcinoma: further evidence for a unified histopathologic grading system for squamous cell carcinomas of the upper aerodigestive tract. Am. J. Surg. Pathol. 43, 303–313 (2019).
Jesinghaus, M. et al. Introducing a novel highly prognostic grading scheme based on tumour budding and cell nest size for squamous cell carcinoma of the uterine cervix. J. Pathol. Clin. Res. 4, 93–102 (2018).
Zare, S. Y., Aisagbonhi, O., Hasteh, F. & Fadare, O. Independent validation of tumor budding activity and cell nest size as determinants of patient outcome in squamous cell carcinoma of the uterine cervix. Am. J. Surg. Pathol. 44, 1151–1160 (2020).
Satabongkoch, N. et al. Prognostic value of tumor budding in early-stage cervical adenocarcinomas. Asian. Pac. J. Cancer Prev. 18, 1717–1722 (2017).
Bhatla, N., Aoki, D., Sharma, D. N. & Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynaecol. Obstet. 143, 22–36 (2018).
Barresi, V. et al. Histologic grading based on counting poorly differentiated clusters in preoperative biopsy predicts nodal involvement and pTNM stage in colorectal cancer patients. Hum. Pathol. 45, 268–275 (2014).
Pirog, E. C. et al. Gastric-type adenocarcinoma of the cervix: tumor with wide range of histologic appearances. Adv. Anat. Pathol. 26, 1–12 (2019).
Roma, A. A. et al. New pattern-based personalized risk stratification system for endocervical adenocarcinoma with important clinical implications and surgical outcome. Gynecol. Oncol. 141, 36–42 (2016).
Maffeis, V., Nicolè, L. & Cappellesso, R. RAS, Cellular plasticity, and tumor budding in colorectal cancer. Front. Oncol. 9, 1255 (2019).
Tommasino, M. et al. The role of TP53 in cervical carcinogenesis. Hum. Mutat. 21, 307–312 (2003).
Jesinghaus, M. et al. Morphology matters: a critical reappraisal of the clinical relevance of morphologic criteria from the 2019 WHO classification in a large colorectal cancer cohort comprising 1004 cases. Am. J. Surg. Pathol. 45, 969–978 (2021).
Jun, S. Y. et al. Tumor budding and poorly differentiated clusters in small intestinal adenocarcinoma. Cancers (Basel) 12, 2199 (2020).
Lohneis, P. et al. Tumor budding assessed according to the criteria of the International Tumor Budding Consensus Conference determines prognosis in resected esophageal adenocarcinoma. Virchows Arch. 478, 393–400 (2021).
O’Connor, K. et al. Tumor budding is an independent adverse prognostic factor in pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 39, 472–478 (2015).
Li, X., Wei, B., Sonmez, C., Li, Z. & Peng, L. High tumor budding count is associated with adverse clinicopathologic features and poor prognosis in breast carcinoma. Hum. Pathol. 66, 222–229 (2017).
Wei, L. et al. A classification based on tumor budding and immune score for patients with hepatocellular carcinoma. Oncoimmunology 9, 1672495 (2019).
Yamaguchi, Y. et al. Histopathologic features of the tumor budding in adenocarcinoma of the lung: tumor budding as an index to predict the potential aggressiveness. J. Thorac. Oncol. 5, 1361–1368 (2010).
Park, J. Y., Hong, D. G., Chong, G. O. & Park, J. Y. Tumor budding is a valuable diagnostic parameter in prediction of disease progression of endometrial endometrioid carcinoma. Pathol. Oncol. Res. 25, 723–730 (2019).
Ueno, H. et al. New criteria for histologic grading of colorectal cancer. Am. J. Surg. Pathol. 36, 193–201 (2012).
Barresi, V. et al. Colorectal carcinoma grading by quantifying poorly differentiated cell clusters is more reproducible and provides more robust prognostic information than conventional grading. Virchows Arch. 461, 621–628 (2012).
Baalbergen, A., Ewing-Graham, P. C., Hop, W. C., Struijk, P. & Helmerhorst, T. J. Prognostic factors in adenocarcinoma of the uterine cervix. Gynecol. Oncol. 92, 262–267 (2004).
Chargui, R. et al. Prognostic factors and clinicopathologic characteristics of invasive adenocarcinoma of the uterine cervix. Am. J. Obstet. Gynecol. 194, 43–48 (2006).
Acknowledgements
We thank Mrs. Minhua Yu for her excellent technical support. We also thank Dr Brian Eyden, from Manchester, UK, for his kind help in linguistic refinement of this paper.
Funding
This work is supported by a grant from the National Natural Scientific Foundations of China (81872112) (B. Lu).
Author information
Authors and Affiliations
Contributions
H.S. and Y.L. performed the study and data collection. H.S. also wrote the paper. W.L. performed data analysis, and review and revision of the paper. B.L. performed study concept and design, and approved the final submission. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study was approved by the Institutional Review Board, Women’s Hospital, School of Medicine, Zhejiang University (IRB: 20170139). The study was performed in accordance with the Declaration of Helsinki.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shi, H., Ye, L., Lu, W. et al. Grading of endocervical adenocarcinoma: a novel prognostic system based on tumor budding and cell cluster size. Mod Pathol 35, 524–532 (2022). https://doi.org/10.1038/s41379-021-00936-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00936-1