Abstract
The College of American Pathologists/American Society of Clinical Oncology recommends HER2 testing prior to initiation of targeted therapy for patients with advanced Gastroesophageal adenocarcinoma (GEA), using immunohistochemistry (IHC) followed by fluorescence in situ hybridization (FISH) in cases with an equivocal (score 2 + ) result on IHC. The FISH results are considered indeterminate if the HER2/CEP17 ratio is <2.0 with an average CEP17 copy number of ≥3.0 and a HER2 copy number ≥4.0 and ≤6.0 after counting additional tumor cells. Indeterminate results may be resolved by using an alternative chromosome 17 probe such as RAI1. The purpose of this study is to review our experience with RAI1 alternate probe in HER2 FISH testing of GEA in a large reference laboratory setting. Esophageal, gastroesophageal, and gastric adenocarcinomas received for HER2 FISH testing in our lab between 9/2018 and 1/2020 were included. HER2/CEP17 and HER2/ RAI1 ratios, and the average HER2, CEP17, RAI1 signals per cell were recorded. 328 GEA had HER2 testing performed in our lab during the study period. 101 (30.8%) were amplified, 169 (51.5%) were non-amplified and 58 (17.7%) were indeterminate. Following RAI1 testing, 42 (72.4%) of 58 indeterminate cases were reclassified as non-amplified and 16 (27.6%) were reclassified as amplified, increasing the total amplified cases to 117 (35.7%). The correlation between the average CEP17 and RAI1 copy number for all cases was weak (R2 = 0.095). In summary, using the alternate probe RAI1 reclassifies 27.6% of original HER2 FISH indeterminate gastroesophageal carcinomas as amplified, which makes them eligible for targeted therapies.
Similar content being viewed by others
Introduction
Gastroesophageal adenocarcinoma (GEA) is the fifth most frequently diagnosed cancer and the third leading cause of cancer-related death worldwide. ERBB2 (erb-b2 receptor tyrosine kinase 2 or HER2) is a biomarker used to identify eligible patients for specific therapy in the setting of advanced GEA. HER2 overexpression is seen in 12–30% of GEA with no significant differences between primaries and metastases1,2. Currently the standard of practice is to perform HER2 testing on all patients with advanced GEA who are candidates for HER2-targeted therapy, regardless of histologic subtype, specimen type (biopsy or resection), differentiation and precise tumor site (stomach, gastroesophageal junction, esophagus or metastatic deposit). Patients with positive results on HER2 testing are offered targeted therapy such as Trastuzumab, which is a monoclonal antibody targeting the HER2 receptor. Trastuzumab reduces proliferation, suppresses angiogenesis and promotes cell death through antibody dependent cell mediated cytotoxicity3. Improved survival has been documented in the randomized controlled trail ToGA (Trastuzumab for Gastric Cancer) for GEA patients treated with Trastuzumab alongside with chemotherapeutic agents, further emphasizing the importance of accurate HER2 testing and reporting4.
The College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology (CAP/ASCO) have published comprehensive guidelines on HER2 testing in GEA based on literature review and consensus opinion, which differ from the guidelines established for breast cancer5,6. Notably, HER2 overexpression in GEA may be more heterogeneous and the staining pattern may be more basolateral rather than circumferential on immunohistochemical stains (IHC). In addition, IHC patterns observed in cancer cell clusters consisting of ≥5 neoplastic cells in biopsies (rather than required 10% of the tumor cells in GEA excisions, or any breast tumors) is considered reactive5. IHC is the recommended primary test of choice and no further testing is indicated for negative (scores 0 or 1 + ) or positive (score 3 + ) cases for GEA. All tumors with equivocal scores (2 + ) on initial IHC analysis are reflexed to in situ hybridization for determining the amplification status. Both FISH and bright field ISH are acceptable means to assess for HER2 amplification, and reported as positive when HER2/CEP17 ratio is ≥2.0 or average HER2 signal number per cell is >6.0 and negative when HER2/CEP17 ratio is <2.0 when average HER2 signal number per cell is <6.0 and average CEP17 signal number per cell is <3.0. Cases are deemed indeterminate if HER2/CEP17 ratio is <2.0 with an average ≥4 to ≤6 HER2 signals per cell, and average CEP17 signal number of ≥3.0 even after counting an additional 20 tumor cells. Extra (≥3) copies of CEP17 were noted in 4% of gastric cancers which is the result of intrachromosomal segmental duplication overlapping the centromere of chromosome 17 also involving the HER2 gene5. The 2016 CAP/ASCO guidelines, offers additional options for indeterminate test results including selecting a different block, retesting with an alternative chromosome 17 probe (instead of CEP17), among other measures such as using genomics or alternative testing5.
Retinoic acid-induced 1 gene (RAI1) has been used as alternative probe to resolve indeterminate FISH results using HER2 and CEP17 probes7,8. RAI1 is located on the short arm of chromosome 17 (band position 17p11.2) and has a reported low coamplification rate of about 1%, and therefore serves as an attractive alternative locus for HER2 FISH testing8,9. However, some studies describe frequent deletion in 17p11.2, which could potentially result in overestimating the frequency of amplified cases9,10. Much of our current understanding on using RAI1 as a probe in HER2 testing is derived from studies done in breast cancer, although the HER2 testing in breast cancer no longer recommend using alternative probes to resolve indeterminate FISH results6,11. Despite that fact, alternative probes such as RAI1 remain within testing algorithms for GEA samples5. In a reference laboratory setting, alternate chromosome 17 probe has been best option for resolving indeterminate HER2 FISH in GEA cases where presence/absence of another tissue block is unknown. The purpose of this study is to do a retrospective analysis and describe our experience with using RAI1 as an alternative probe locus in the HER2 testing algorithm for GEA.
Materials and methods
The study protocol was approved by the University of Utah Institutional Board Review (IRB#77507). A search was conducted using our laboratory information system for samples submitted for HER2 testing by immunohistochemistry (IHC) with reflex to HER2 FISH or submitted primarily for HER2 FISH testing. Cases were analyzed at ARUP Laboratories, which serves as a national reference laboratory. Only primary esophageal, gastroesophageal, and gastric adenocarcinomas as designated on the accompanying test requisition and received between September 2018 and January 2021 were included in our study cohort. Metastatic tumors were excluded from the analysis as tumor origin was considered to be uncertain due to limited information on the pathology reports provided by the outside institutions.
Hematoxylin-eosin-stained slides were reviewed to confirm the presence and extent of tumor tissue from both biopsies and resections. Immunohistochemical stain analysis was carried out with HercepTest (Dako, Carpinteria, California) following ASCO/CAP 2016 guidelines for HER2 testing of GEA5 and scored manually by board certified anatomic pathologists using the Ruschoff/Hofmann method12. For biopsy specimens the interpretation was negative (score of 0 or 1 + ) if there was no reactivity or faint barely perceptible membranous reactivity, equivocal (score of 2 + ) if there were ≥5 tumor cell clusters with weak to moderate complete, basolateral, or lateral membranous reactivity irrespective of percentage of stained tumor cells. Biopsies were interpreted as positive (score of 3 + ) if there were ≥5 tumor cell clusters with strong complete, basolateral or lateral membranous reactivity irrespective of percentage of stained tumor cells. HER2 IHC scoring was similar for the resection specimens, but a threshold of 10% was used in the assessment. Cases with an equivocal (or score of 2 + ) result on IHC analysis were reflexed to HER2 analysis by FISH.
HER2 FISH analysis was performed using the US Food and Drug Administration (FDA)-approved, dual-probe HER2 IQFISH (Dako, Carpinteria, California). The FDA-approved probe set was scored according to the ASCO/CAP 2016 guidelines for interpreting GEA HER2 FISH assays. In summary a GEA was reported as non-amplified/negative if the HER2/CEP17 ratio was <2.0, HER2 copy number per cell ≤6.0 and average CEP17 copy number per cell <3.0, amplified/positive if HER2/CEP17 ratio ≥2.0, or HER2 copy number per cell >6.0 regardless of the HER2/CEP17 ratio, or indeterminate if HER2/CEP17 ratio is <2.0 with an average CEP17 copy number per cell ≥3.0 and HER2 copy number per cell ≥4.0 and ≤6.0. At least 20 cells were counted in each case and reviewed by both a technician and a board-certified pathologist trained in anatomic pathology. A minimum of an additional 20 cells were counted if the result was close to the cutoff, if there was significant cell to cell variability in the number of signals, or if the results were indeterminate. The indeterminate cases were retested with HER2 and RAI1 probes (both from Agilent Technologies, Santa Clara, California) and interpreted as non-amplified if the HER2/RAI1 ratio was <2.0 and the average number of RAI1 per cell was 1.5 or greater, or as amplified if HER2/RAI1 ratio was 2.0 or more. HER2 status was considered unresolved or indeterminate with a suggestion for additional/alternative testing for cases with a deleted RAI1 (defined as a RAI1 copy number per cell of <1.5). Concurrent copy number gains in HER2, the centromere, and RAI1 were assumed to be unlikely representation of true HER2 amplification, and therefore there were no established cutoffs for RAI1 gains as these cases were more likely to represent polysomy rather than co-amplification. Our laboratory participates in CAP’s GEA HER2 IHC, and HER2 FISH proficiency testing. All HER2 immunohistochemical tests have on slide, and FISH tests have batch positive controls.
The results of previous HER2 immunohistochemical testing (if performed in our laboratory) and FISH data with the FDA approved probe set and the alternate probe set were retrieved from electronic files. The average copy number per cell of HER2, CEP17, HER2, and RAI1 from each probe set and their corresponding ratios were recorded.
Results
There were 330 gastric, gastroesophageal and esophageal adenocarcinoma specimens identified that met the search criteria. Two of 330 cases did not have sufficient tissue on FISH slides and were excluded from further analysis. Only one case (0.03%) was received at our laboratory for immunohistochemical testing followed by reflex FISH testing if equivocal (2 + ). We have performed HER2 IHC testing on two additional cases. The initial testing with the HER2 FISH FDA approved probe set showed that slightly over half of all included cases were classified as non-amplified or negative (n = 169) and about 30% of the cases were classified as amplified or positive (n = 101) using the ASCO/CAP 2016 reporting criteria (Table 1). The indeterminate category retested with the RAI1 alternate probe, constituted approximately 18% of all studied cases (n = 58) (Table 1). These cases have shown a HER2/CEP17 ratio of <2 with an average CEP17 copy number of ≥3.0 and HER2 copy number per cell ≥4.0 and ≤6.0. Sixteen indeterminate cases (out of 58, or 27.6%) were reclassified as amplified after retesting with the RAI1 probe, while 42 cases (out of 58, or 72.4%) were reclassified as non-amplified after retesting with the RAI1 probe (Fig. 1). The results are summarized in Table 1. Of note we did not identify a case with an unresolved HER2 result after retesting with the RAI1 alternate probe in our cohort. Sixteen (27.6%) of the originally indeterminate cases that were classified as amplified after re-testing with alternate RAI1 probe had an average HER2/RAI1 ratio of 2.29 (Table 2). The correlation between average CEP17 and RAI1 copy numbers for these cases was poor (Fig. 2).
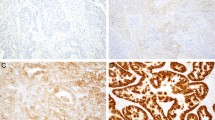
A through D Example of GEA with equivocal HER2 immunohistochemistry (IHC) and HER2 FISH results that was reclassified as amplified by the alternate RAI1 probe with HER2/RAI1 ratio of 2.5. E through H Example of GEA with equivocal HER2 IHC and HER2 FISH results that had a nonamplified HER2/RAI ratio (original magnification, ×40 [A, B, E, and F]; original magnification, ×100 [C, D, G, and H].
Discussion
In our cohort, 58 out of 328 (17.7%) GEA cases required retesting with RAI1 alternate probe set during the study period in order to resolve indeterminate HER2 FISH result after the FDA approved original probe set. To the best of our knowledge, this is the largest described cohort of GEA tested with an RAI1 alternate probe reported in literature. We found that using a RAI1 probe set reclassifies over 27% of original HER2 FISH indeterminate gastroesophageal cancer cases as amplified or positive making them potentially eligible for targeted therapy.
The most recent ASCO/CAP guidelines for GEA permit using alternative FISH probes to resolve indeterminate results on HER2 FISH testing5. However, the ASCO/CAP guideline does not comment on the interpretation of the reporting of alternate probes such as RAI1. Other recommended options to resolve an indeterminate result on FISH include consulting another scorer regarding selection of tumor areas for scoring or using a different tumor block. In our laboratory both a technician and pathologist review all cases for concordance, which has been an integral part of our testing algorithm. Testing a different tumor block is not feasible in the reference laboratory setting because usually only one block is submitted for testing. Also, tumor is frequently present in only one block, particularly in biopsies, so no alternative blocks are usually available for analysis. Using an alternative probe remains the most feasible option to resolve indeterminate HER2 FISH cases in reference laboratory setting, unless genomic analysis becomes more readily available as a reflex test option in the future13,14.
In the past several loci have been evaluated as alternate probe sites for resolving equivocal HER2 FISH results for breast cancer, including RARA (retinoic acid receptor alpha)—17q21.2, RAI1 (retinoid acid-induced 1, previously known as SMS for Smith-Magenis syndrome)—17p11.2, and TP53–17p13.18. The indeterminate HER2 FISH cases have reported an upgrade rate of up to 40% when using RAI1 in breast cancer with 3.1% still unclassifiable due to deletion of RAI1 in a similar, reference laboratory setting11. The deletion of 17p11.2 and chromosome 17 polysomy likely occurs at a different rate in breast and GEAs9,10,14. The deletions in the RAI1 locus are a potential cause of overestimation of amplified cases in breast cancers because the deletion of a control probe would result in ratios in the amplified range, even in cases with low (<4) HER2 copy numbers. Notably, we have not identified any cases with deleted RAI1 probe in our cohort of GEAs. Thus, the deletion in the 17p11.2 region may be less common in GEAs as compared to breast cancers. The poor correlation between CEP17 and RAI1 in cases is linked to the extent and mechanism of copy number changes occurring in given tumors. While true polysomy is unusual in breast carcinoma, the co-amplification of CEP17 and HER2 are usually due to gene amplification encompassing the centromeric or pericentromic region8,15. In contrast, in gastric carcinomas, the mechanism is intrachromosomal segmental duplication of the HER2 and centromeric region16. Furthermore, while evidence remains limited, studies in breast carcinoma show lack of evidence of adverse outcomes in patients with amplified HER2 status based on an alternate probe testing compared to equivocal or negative cases using the CEP17 probes10.
Although some patients with GEA show durable response to targeted therapy, most either show primary or acquired resistance. This may be due to tumor heterogeneity and/or previously undocumented discrepancy between primary tumors and metastatic carcinomas1,17. More recent studies have shown that the level of HER2 amplification correlates with response to trastzumab in patients with metastatic GEA cancers having ratios ≥3.0 or ≥4.01,18. The clinical response to targeted therapy in GEA based on a positive result obtained from RAI1 alternate probe use is currently not known. However, all 16 cases in this cohort that were re-classified as amplified following alternate probe testing had HER2/RAI1 ratio <3.0 (range:2.0–2.8; average; 2.29).
This study was conducted in a large reference laboratory setting, which precluded access to clinical charts in the majority of tested samples. Therefore, prior HER2 testing at the referring institution could not be confirmed in our cohort. Although only one case had an immunohistochemical test followed by reflex FISH testing request ordered, two additional cases had IHC done by our lab. However, it is assumed that the remaining cases had prior HER2 IHC testing performed by the referring institutions following the current HER2 testing guidelines5. Segmental duplication of chromosome 17 with 3 or more copies of CEP17 were noted in 4.1% of gastric cancers in the ToGA trial4,5. The high proportion (17.7%) of indeterminate cases in this study is likely due to referral bias.
Our reference laboratory serves many out of state clients and smaller hospitals, and thus is subject to referral bias. Overall, it is uncertain if cases are representative of all GEA in the general population. We identified that 30.8% and 35.7% of GEA are amplified following testing with FDA approved probe set and additional testing with an alternate probe in indeterminate cases respectively, which falls within the range of published rates of positivity in GEA, reported as 4.4–53.4% with a mean of 17.9%5,19.
In summary, our study showed that RAI1 alternative probe reclassifies more than a quarter of GEA with indeterminate HER2 FISH result as amplified making them eligible for targeted therapy. However, it is largely unknown whether this subgroup of patients will benefit from such therapies.
Data availability
All data analyzed in this study are included in this paper.
References
Haffner, I. et al. HER2 Expression, Test Deviations, and Their Impact on Survival in Metastatic Gastric Cancer: results From the Prospective Multicenter VARIANZ Study. J. Clin. Oncol. 39, 1468–1478 (2021).
Janjigian, Y. Y. et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: a European and USA International collaborative analysis. Ann. Oncol. 23, 2656–2662 (2012).
Hudis, C. A. Trastuzumab-mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Bang, Y. J. et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010).
Bartley, A. N. et al. HER2 Testing and Clinical Decision Making in Gastroesophageal Adenocarcinoma: guideline From the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. Am. J. Clin. Pathol. 146, 647–669 (2016).
Wolff, A. C. et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch. Pathol. Lab. Med. 142, 1364–1382 (2018).
Agersborg, S. et al. Immunohistochemistry and alternative FISH testing in breast cancer with HER2 equivocal amplification. Breast Cancer Res. Treat. 170, 321–328 (2018).
Tse, C. H. et al. Determining true HER2 gene status in breast cancers with polysomy by using alternative chromosome 17 reference genes: implications for anti-HER2 targeted therapy. J. Clin. Oncol. 29, 4168–4174 (2011).
Jang, M. H., Kim, E. J., Kim, H. J., Chung, Y. R. & Park, S. Y. Assessment of HER2 status in invasive breast cancers with increased centromere 17 copy number. Breast Cancer Res. Treat. 153, 67–77 (2015).
Sneige, N., Hess, K. R., Multani, A. S., Gong, Y. & Ibrahim, N. K. Prognostic significance of equivocal human epidermal growth factor receptor 2 results and clinical utility of alternative chromosome 17 genes in patients with invasive breast cancer: a cohort study. Cancer 123, 1115–1123 (2017).
Hui, L., Geiersbach, K. B., Downs-Kelly, E. & Gulbahce, H. E. RAI1 Alternate Probe Identifies Additional Breast Cancer Cases as Amplified Following Equivocal HER2 Fluorescence In Situ Hybridization Testing: Experience From a National Reference Laboratory. Arch. Pathol. Lab. Med. 141, 274–278 (2017).
Hofmann, M. et al. Assessment of a HER2 scoring system for gastric cancer: results from a validation study. Histopathology 52, 797–805 (2008).
Nakamura, K. et al. Estimating copy number using next-generation sequencing to determine ERBB2 amplification status. Med. Oncol. 38, 36 (2021).
Ross, D. S. et al. Next-Generation Assessment of Human Epidermal Growth Factor Receptor 2 (ERBB2) Amplification Status: Clinical Validation in the Context of a Hybrid Capture-Based, Comprehensive Solid Tumor Genomic Profiling Assay. J. Mol. Diagn. 19, 244–254 (2017).
Yeh, I. T. et al. Clinical validation of an array CGH test for HER2 status in breast cancer reveals that polysomy 17 is a rare event. Mod. Pathol. 22, 1169–1175 (2009).
Bass, A. J. et al. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Pectasides, E. et al. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Disco. 8, 37–48 (2018).
Gomez-Martin, C. et al. Level of HER2 gene amplification predicts response and overall survival in HER2-positive advanced gastric cancer treated with trastuzumab. J. Clin. Oncol. 31, 4445–4452 (2013).
Abrahao-Machado, L. F. & Scapulatempo-Neto, C. HER2 testing in gastric cancer: an update. World J. Gastroenterol. 22, 4619–4625 (2016).
Author information
Authors and Affiliations
Contributions
The study was conceptualized and written by J.J. and H.E.G. N.U. provided technical and material support. J.J., D.S., N.U., D.S. reviewed and edited the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The study was approved by the University of Utah Institutional Review Board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jedrzkiewicz, J., Sirohi, D., Uvejzovic, N. et al. RAI1 alternate probe identifies additional gastroesophageal adenocarcinoma cases as amplified following equivocal HER2 fluorescence in situ hybridization testing: experience from a national reference laboratory. Mod Pathol 35, 549–553 (2022). https://doi.org/10.1038/s41379-021-00933-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00933-4