Abstract
Follicular thyroid carcinoma (FTC) has different clinicopathological characteristics than papillary thyroid carcinoma. However, there are no independent systems to predict cancer-specific survival (CSS) in FTC. Telomerase reverse transcriptase (TERT) promoter mutations are associated with tumor aggressiveness. Thus, it could be a potential prognostic marker. The aim of this study was to refine the CSS risk prediction using TERT promoter mutations in combination with the fourth edition of World Health Organization (WHO 2017) morphological classification. We investigated 77 FTC patients between August 1995 and November 2020. Cox regression was used to calculate hazard ratios to derive alternative groups. Disease-free survival (DFS) and CSS predictability were compared using Proportion of variation explained (PVE) and C-index. CSS was significantly different in encapsulated angioinvasive (EA)-FTC patients stratified by TERT promoter mutations [wild-type (WT-TERT) vs. mutant (M-TERT); P < 0.001] but not in minimally invasive (MI)-FTC and widely invasive (WI)-FTC patients (P = 0.691 and 0.176, respectively). We defined alternative groups as follows: Group 1 (MI-FTC with WT-TERT and M-TERT; EA-FTC with WT-TERT), Group 2 (WI-FTC with WT-TERT), and Group 3 (EA-FTC with M-TERT; WI-FTC with M-TERT). Both PVE (22.44 vs. 9.63, respectively) and C-index (0.831 vs. 0.731, respectively) for CSS were higher in the alternative groups than in the WHO 2017 groups. Likewise, both PVE (27.1 vs. 14.9, respectively) and C-index (0.846 vs. 0.794, respectively) for DFS were also higher in the alternative groups than in the WHO 2017 groups. Alternative group harmonizing of the WHO 2017 classification and TERT promoter mutations is effective in predicting CSS in FTC patients, thereby improving DFS predictability.
Similar content being viewed by others
Introduction
Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) are both derived from the follicular epithelium of the thyroid gland, and they have the ability to concentrate radioactive iodine1. Due to this similarity, both PTC and FTC are usually considered differentiated thyroid carcinomas (DTCs). Since FTC occurrence is less common than that of PTC2, staging and treatment strategies for DTC are primarily focused on PTC3. However, PTC and FTC have markedly different epidemiological, cytological, pathological, genetic, and clinical behavioral characteristics4,5,6,7,8,9,10,11.
The World Health Organization (WHO) classification of tumors serves as an international standard for histopathological diagnosis. In a previous WHO classification (WHO 2004), FTCs were divided into minimally invasive and widely invasive types12. However, important modifications to the classification of FTC were made in the revised fourth edition of the WHO classification (WHO 2017)13. FTCs are now divided into three categories on the basis of the invasive pattern and angioinvasion: minimally invasive (MI-FTC), encapsulated angioinvasive (EA-FTC), and widely invasive (WI-FTC). After the WHO staging system was revised in 2017, 20.4% of FTC patients were re-classified from MI-FTC to EA-FTC. The predictability of disease-free survival (DFS) has improved as a result of this change but not that of cancer-specific survival (CSS)14.
A number of risk stratification and staging systems have been propounded with Lang B. H. et al. reporting that the AJCC/TNM system has the best predictability of CSS in FTC patients15. However, these stratification systems were developed mainly for patients with PTC, and there is no specific staging system to predict CSS in FTC patients.
Recent studies have identified telomerase reverse transcriptase (TERT) promoter mutations that are closely associated with tumor aggressiveness, early recurrence, and cancer specific deaths in patients with thyroid cancer16,17,18,19. Though these potential prognostic markers are very promising, none of the current recurrence or mortality risk systems incorporate molecular testing results in thyroid cancer stratification. Recently, molecular marker-based risk stratification of thyroid cancer has been proposed to better predict the clinical outcome of the cancer20,21,22. In this study, we refined risk prediction for thyroid cancer using TERT promoter mutations and WHO 2017 morphological classification to enhance CSS and DFS predictions.
Methods
Study population
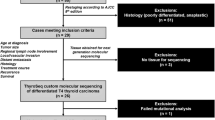
From August 1995 to November 2020, 82 consecutive FTC patients who had undergone initial thyroid surgery at Samsung Medical Center and showed TERT promotor mutations, as determined by DNA sequencing, were enrolled. Of the 82 patients, we excluded four patients with follicular variant PTC (FV-PTC) and one patient with PTC. Among 77 patients, 59 were female, and 18 were male. Patients with Hürthle cell thyroid carcinoma and poorly differentiated thyroid carcinoma were not included in this study. This study was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2021-04-085). Informed consent was waived by the committee as it was a retrospective study.
Clinicopathological data and outcomes
Operating records and final pathologic reports were reviewed to ascertain tumor categories based on the WHO 2017 classification and the eighth edition of the AJCC/TNM classification (TNM-8). In TNM-8, tumors invading strap muscles, subcutaneous soft tissue, larynx, trachea, esophagus, recurrent laryngeal nerve, and prevertebral fascia, or encasing the carotid artery or mediastinal vessel, are classified as gross extrathyroidal extension (ETE). A pathologist (Y.L.O.) at the Department of Pathology reviewed the pathology slides of patients with multifocality or cervical lymph node metastasis to exclude the possibility of misdiagnoses, such as FV-PTC. The status of vascular invasion was also pathologically confirmed, and all patients were reclassified in accordance with the WHO 2017 criteria into one of the following categories: MI-FTC, EA-FTC, and WI-FTC23.
DFS was defined as the time from initial surgery to the date of the first structural recurrence. Structural recurrence was defined as persistent or recurrent disease, determined cytologically or pathologically, and/or the presence of highly suspicious metastatic lesions as observed by imaging. CSS was defined as the time from initial surgery to the time of death due to thyroid cancer. Data of patients who died due to other causes were censored at the time of death.
Detection of TERT promotor mutation
Promotor mutations in TERT were identified by semi-nested polymerase chain reaction (PCR) and direct Sanger sequencing of the hot spots (chr5:1,295,228 C > T and chr5:1,295,250 C > T) commonly termed C228T and C250T as previously described24,25,26.
Statistical analysis
Continuous variables were presented as mean with standard deviation (SD), and categorical variables were presented as numbers and percentages. Patients were stratified as per the WHO 2017 classification and the TERT promoter mutation status. Cox regression analysis was used to calculate unadjusted hazard ratios (HRs) to predict the outcome of CSS and DFS, thereby deriving alternative prognostic groupings. Survival curves were plotted using the Kaplan–Meier method, and the log-rank test was used to compare survival significance. To estimate the relative validity of predicting CSS and DFS in each of the WHO 2017 categories and the alternative groups, we calculated the proportions of variation explained (PVEs) using the Cox proportional regression model and Harrell’s C-index21,27,28. The PVEs (%) range from 0 to 100 with higher percentages indicating better predictability. The maximum value of the C-index was 1.00, and higher values indicated a more accurate predictive capacity. Statistical analysis was executed using R 4.0.4 (Vienna, Austria; http://www.R-project.org/), and SPSS version 25.0 for Windows (IBM, Chicago, IL, USA).
Results
Clinical characteristics
A total of 77 patients were included in this study; 39 patients with MI-FTC, 24 patients with EA-FTC, and 14 patients with WI-FTC. The baseline clinicopathological characteristics according to the WHO 2017 classification are described in Table 1. The presence of gross ETE (P for trend = 0.023), presence of distant metastasis (P for trend <0.001), status of TERT promoter mutations (P for trend = 0.033), and AJCC/TNM stage (P for trend <0.001) were significantly associated with the aggressiveness of the pathological characteristics in the WHO 2017 classification. Sex, age, and primary tumor size were not significantly different between WHO 2017 groups.
Prognostic outcomes according to the WHO 2017 classification and TERT promoter mutations
We evaluated CSS in terms of the presence of TERT promoter mutations in the three WHO-2017 groups. Most notably, TERT promoter mutations were significantly associated with CSS only in the EA-FTC patients (P < 0.001) (Fig. 1b). CSS did not differ in the presence of TERT promoter mutations in the MI-FTC and WI-FTC patients (Fig. 1a, c). When the patients were stratified according to the WHO 2017 classification system and TERT promoter mutational status, the HRs of CSS were found to be higher in the EA-FTC patients with M-TERT (HR: 59.09; 95% CI: 5.72–610.68), and the WI-FTC patients with M-TERT (HR 23.26; 2.33–231.78), whereas there were no MI-FTC patients with M-TERT who died of FTC. In patients with WT-TERT, the HRs of CSS increased with increasing pathological aggressiveness as mentioned in the WHO 2017 classification system (Supplementary Table 1).
We also evaluated DFS against the status of TERT promoter mutations in the three WHO 2017 groups. In the EA- and WI-FTC patients, DFS was significantly different with differing TERT promoter mutational status (P = 0.004 and P = 0.020, respectively) but not in the MI-FTC patients (P = 0.466) (Fig. 2). The pattern of HRs of DFS in the six categories was similar to that of CSS (Supplementary Table 2).
After running a comparison among the six categories, we produced the following three alternative groups: Group 1 (MI-FTC with WT-TERT and M-TERT; EA-FTC with WT-TERT), Group 2 (WI-FTC with WT-TERT), and Group 3 (EA-FTC with M-TERT; WI with M-TERT) (Fig. 3). The clinicopathological characteristics between the three alternative groups are shown in Supplementary Table 3. The presence of gross ETE (P for trend <0.001), presence of distant metastasis (P for trend <0.001), and AJCC/TNM stage (P for trend <0.001) were significantly different between the groups.
Cancer-specific survival according to alternative groups
Of the 77 patients, 11 patients died of FTC at a median of 14.8 (interquartile range 1.6–19.7 years) years after the initial operation. Among the WHO 2017 groups, 15-year CSS rates for MI-, EA-, and WI-FTC patients were found to be 95.5, 78.3, and 55.6%, respectively (P = 0.015) for which the Kaplan–Meier analysis is shown in Fig. 4a. However, among the alternative groups, the 15-year CSS rates for group 1, 2, and 3 patients were found to be 94.6, 66.7, and 18.8%, respectively (P < 0.001) for which the Kaplan–Meier survival curve is shown in Fig. 4b. Table 2 shows the HRs of CSS in the WHO 2017 and alternative groups. Groups 2 (HR 7.09; 95% CI 1.18–42.46) and 3 (HR 32.69; 95% CI 6.25–170.84) showed significantly higher HRs than group 1 (P = 0.032 and P < 0.001, respectively). The PVEs were 22.44 for the alternative groups and 9.63 for the WHO 2017 groups. The C-index was also higher in the alternative groups than in the WHO 2017 groups (0.831 vs. 0.731, respectively).
Disease-free survival according to alternative groups
Figure 5 shows the Kaplan–Meier survival curve for DFS. The 15-year DFS rates for the MI-, EA-, and WI-FTC groups were 90.2, 85.6, and 42.9%, respectively (P < 0.001). Among the alternative groups, the 15-year DFS rates for groups 1, 2, and 3 were 91.7, 66.7, and 0.0%, respectively (P < 0.001). The HRs of DFS in each of the WHO 2017 and alternative groups are shown in Table 3. Groups 2 and 3 were significantly associated with an increased risk of disease recurrence as compared to group 1 (P = 0.037, and P < 0.001, respectively). The PVEs in the alternative groups and the WHO 2017 groups were 27.1 and 14.9, respectively. The C-index was also higher in the alternative group than in the WHO 2017 group (0.846 vs. 0.794, respectively).
Discussion
The purpose of this study was to assess whether TERT promotor mutation can be a new molecular prognostic marker for predicting disease specific survival in FTC patients. We found that the presence of TERT promoter mutations was significantly associated with poor survival in the EA-FTC group. Thus, we defined three patient groups based on the WHO 2017 morphological classification and the presence of TERT promoter mutations. During the median follow-up of 14.8 years, the HRs of CSS significantly increased in groups 2 and 3, whereas the HRs were not significantly different between the MI-FTC and EA-FTC WHO 2017 groups. Furthermore, the PVE and C-index of CSS were higher in the alternative groups than in the WHO-2017 groups, which suggested that the alternative group had better predictability for CSS in patients with FTC. Illustrations of each of three WHO 2017 classification with or without TERT promoter mutation were shown in Fig. 6.
a Minimally invasive (MI)-FTC with wild-type TERT (WT-TERT), b MI-FTC with mutant TERT (M-TERT), c encapsulated angioinvasive (EA)-FTC with WT-TERT, d EA-FTC with M-TERT, e widely invasive (WI)-FTC with WT-TERT, f WI-FTC with M-TERT, g a high magnification of WI-FTC with WT-TERT, h a high magnification of WT-FTC with M-TERT showing focal insular pattern with rare mitotic figures.
TERT promoter mutations are associated with enhanced telomere maintenance, and cancer cells can be immortalized by maintaining the telomere length29. Previous studies have shown the association that TERT promoter mutations are associated with tumor aggressiveness and patients survival in DTC16,17,18,21,30,31. The BRAFV600E mutation is considered to be a poor prognostic molecular marker of PTC20. Furthermore, only one case of a patient with FTC carrying a BRAFK601E mutation has been reported8. Despite these differences, previous studies evaluated FTC to be considered as DTC, and most of the results from thyroid cancer were predominantly PTC. Considering that FTC showed poorer survival outcome than PTC, and that both have different clinical and molecular characteristics, independent risk-group stratification is needed.
Although the WHO 2017 classification of FTC is well-accepted and has improved prognostication by incorporating the importance of vascular invasion, its prognostic implications in CSS are still controversial. For the EA-FTC patients in this study, CSS differed significantly depending on the TERT promoter mutations (log-rank P < 0.001). Thus, we harmonized the WHO 2017 classification and TERT promoter mutations, which are promising molecular prognostic markers, and re-classified the thyroid carcinomas into three alternative groups. Considering that the WI-FTC presented with the most aggressive histology, it was interesting to see that the WI-FTC with WT-TERT patients (group 2) showed better clinical outcome than the EA-FTC with M-TERT patients (group 3). Furthermore, the proportion of TERT promoter mutations varied between the MI-, EA-, and WI-FTC groups (10.3, 20.8, and 35.7%, respectively). Therefore, we believe that the presence of vascular invasion may reflect the aggressiveness of TERT promoter mutations.
Although the alternative groups were proposed to optimize CSS prediction, they also assisted in the prediction of structural recurrence. O’Neil et al. reported that the 10-year DFS of MI-, EA-, and WI-FTC patients was 97, 81, and 46%, respectively32. Likewise, in the present study, 10-year DFS was 95.2, 85.6, and 42.9% in the MI-, EA-, and WI-FTC groups, respectively. However, the PVEs for the WHO 2017 groups were lower than those for the alternative groups. The PVE for DFS was 27.1 in the alternative groups, and the discrimination of HRs was increased in the alternative groups. Notably, there was no distinction between the MI- and EA-FTC WHO 2017 groups (P = 0.587), whereas significant distinctions were observed between the alternative groups 1 and 2 (P = 0.037). Given the favorable outcomes in the majority of FTC patients, identifying patients with a poor expected prognosis is a priority in clinical practice.
This study has several limitations. First, this study is retrospective in nature and was conducted in a single tertiary referral center. Thus, it is prone to selection bias. Second, this study was conducted with a relatively small number of patients, because the prevalence of FTC is relatively low in iodine-sufficient areas of South Korea. Therefore, external validation is encouraged using large population data sets. However, there were no previous reports about long-term follow-up data on TERT promoter mutations as prognostic marker in patients with FTC.
In conclusion, the alternative groups show clinical implications for CSS in patients with FTC. Currently, none of the mortality risk systems incorporate molecular markers as prognostic factors in thyroid carcinoma, even though new robust molecular classifications have been proposed for other cancers33,34. This study demonstrated that promising new molecular prognostic markers can be incorporated into the WHO 2017 classification system to better predict CSS as well as to increase DFS predictability. The results obtained in the present study suggest that TERT promoter mutation tests should be performed in patients with histologically confirmed EA- or WI-FTC.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
Cabanillas, M. E., McFadden, D. G. & Durante, C. Thyroid cancer. Lancet 388, 2783–2795 (2016).
Aschebrook-Kilfoy, B., Ward, M. H., Sabra, M. M. & Devesa, S. S. Thyroid cancer incidence patterns in the United States by histologic type, 1992-2006. Thyroid 21, 125–134 (2011).
Haugen, B. R. et al. American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the american thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26, 1–133 (2016).
Feldt-Rasmussen, U. Iodine and cancer. Thyroid 11, 483–486 (2001).
Cibas, E. S. & Ali, S. Z. The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 27, 1341–1346 (2017).
Kakudo, K. et al. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol. Int. 68, 641–664 (2018).
Nikiforova, M. N. et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 88, 5399–5404 (2003).
Pennelli, G. et al. BRAF(K601E) mutation in a patient with a follicular thyroid carcinoma. Thyroid 21, 1393–1396 (2011).
Xing, M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer 12, 245–262 (2005).
Sciuto, R. et al. Natural history and clinical outcome of differentiated thyroid carcinoma: a retrospective analysis of 1503 patients treated at a single institution. Ann. Oncol. 20, 1728–1735 (2009).
Vogrin, A., Besic, H., Besic, N. & Music, M. M. Recurrence rate in regional lymph nodes in 737 patients with follicular or Hürthle cell neoplasms. Radiol. Oncol. 50, 269–273 (2016).
DeLellis, R. A., Lloyd, R. V., Heitz, P. U. & Eng, C. Pathology and Genetics of Tumours of Endocrine Organs (IARC, 2004).
Protocol for the Examination of Specimens From Patients With Carcinomas of the Thyroid Gland. College of American Pathologist, [Internet], 2017, [cited 20 May 2021]. Available from https://documents.cap.org/protocols/cp-thyroid-17protocol-4000.pdf.
Jin, M. et al. Clinical implication of World Health Organization classification in patients with follicular thyroid carcinoma in South Korea: a multicenter cohort study. Endocrinol. Metab. 35, 618–627 (2020).
Lang, B. H., Lo, C. Y., Chan, W. F., Lam, K. Y. & Wan, K. Y. Staging systems for follicular thyroid carcinoma: application to 171 consecutive patients treated in a tertiary referral centre. Endocr. Relat. Cancer 14, 29–42 (2007).
Kim, T. H. et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer 23, 813–823 (2016).
Liu, X. et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 20, 603–610 (2013).
Melo, M. et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 99, E754–E765 (2014).
Liu, C. et al. TERT promoter mutation and its association with clinicopathological features and prognosis of papillary thyroid cancer: a meta-analysis. Sci. Rep. 6, 36990 (2016).
Xing, M., Haugen, B. R. & Schlumberger, M. Progress in molecular-based management of differentiated thyroid cancer. Lancet 381, 1058–1069 (2013).
Kim, T. H. et al. Refining dynamic risk stratification and prognostic groups for differentiated thyroid cancer with TERT promoter mutations. J. Clin. Endocrinol. Metab. 102, 1757–1764 (2017).
Song, Y. S. et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 122, 1370–1379 (2016).
Lloyd, R. V., Osamura, R. Y., Klöppel, G. & Rosai, J. WHO Classification of Tumours of Endocrine Organs 4th edn (IARC, 2017).
Huang, F. W. et al. Highly recurrent TERT promoter mutations in human melanoma. Science 339, 957–959 (2013).
Lin, Y. S. et al. Long-term results of a Phase II trial of apatinib for progressive radioiodine refractory differentiated thyroid cancer. J. Clin. Endocrinol. Metab. 106, e3027–e3036 (2021).
Sohn, S. Y. et al. Highly concordant key genetic alterations in primary tumors and matched distant metastases in differentiated thyroid cancer. Thyroid 26, 672–682 (2016).
Harrell, F. E. Jr., Lee, K. L. & Mark, D. B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 15, 361–387 (1996).
Schemper, M. & Stare, J. Explained variation in survival analysis. Stat. Med. 15, 1999–2012 (1996).
Vinagre, J. et al. Telomerase promoter mutations in cancer: an emerging molecular biomarker? Virchows Arch 465, 119–133 (2014).
Bournaud, C. et al. TERT promoter mutations identify a high-risk group in metastasis-free advanced thyroid carcinoma. Eur. J. Cancer 108, 41–49 (2019).
Landa, I. et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 98, E1562–E1566 (2013).
O’Neill, C. J. et al. Management of follicular thyroid carcinoma should be individualised based on degree of capsular and vascular invasion. Eur. J. Surg. Oncol. 37, 181–185 (2011).
Eckel-Passow, J. E. et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N. Engl. J. Med. 372, 2499–2508 (2015).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221 (2016).
Funding
The authors received no specific funding for this work.
Author information
Authors and Affiliations
Contributions
T.H.K. and Y.L.O. performed study concept and design. H.P. performed development of methodology and writing, review and revision of the paper. H.C.S., H.Y., J.H., J.H.K., Y.J.C., S.W.K., and J.H.C. provided acquisition, analysis, and interpretation of data. H.P. and H.S.K. did the statistical analysis. C.S.K. and S.Y.H. provided technical and material support. All authors read and approved the final paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The study design was approved by the Institutional Review Board of Samsung Medical Center (IRB no. 2021-04-085). Informed consent was waived by the committee as it was a retrospective study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Park, H., Shin, H.C., Yang, H. et al. Molecular classification of follicular thyroid carcinoma based on TERT promoter mutations. Mod Pathol 35, 186–192 (2022). https://doi.org/10.1038/s41379-021-00907-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00907-6
This article is cited by
-
Genetic landscape of 482 thyroid carcinomas: analysis with the national datacenter for cancer genomic medicine in Japan
Endocrine (2024)
-
Pathogenesis of cancers derived from thyroid follicular cells
Nature Reviews Cancer (2023)
-
High-Grade Non-Anaplastic Thyroid Carcinomas of Follicular Cell Origin: A Review of Poorly Differentiated and High-Grade Differentiated Carcinomas
Endocrine Pathology (2023)
-
DICER1 Mutations Do Not Always Indicate Dismal Prognosis in Pediatric Poorly Differentiated Thyroid Carcinomas
Endocrine Pathology (2023)
-
Molecular pathology of endocrine gland tumors: genetic alterations and clinicopathologic relevance
Virchows Archiv (2023)