Abstract
Despite extraordinary advances in the molecular characterization of soft tissue tumors as a result of the widespread application of next generation sequencing in clinical practice, a subset of lesions remain difficult to diagnose. In this study we describe 3 unclassified spindle cell sarcomas with a monomorphic cytomorphology and distinctive storiform growth, characterized by novel fusions between EWSR1 or FUS1, and NACC1 genes. The tumors occurred in 3 young adult females (age range: 29–31) involving deep soft tissues, two located in the lower extremity and one in the abdominal wall. All three tumors showed patchy positivity for S100 protein, while being negative for SOX10 and retained H3K27me3 expression. All cases were negative for epithelial or muscle markers. As the findings were non-specific, molecular studies using targeted panels of RNA sequencing were performed, including one case tested by TruSight RNA Fusion Panel and 2 cases by Archer FusionPlex. The results showed 2 cases were positive for FUS-NACC1 and one for EWSR1-NACC1 fusions. These findings were further confirmed by FISH using custom BAC probes for a dual-color fusion assay. These results suggest the possibility of a previously undescribed soft tissue neoplasm characterized by a uniform spindle cell phenotype arranged in a storiform and fascicular pattern, expressing S100 protein and harboring NACC1-related fusions. The biologic behavior of this tumor remains to be determined.
Similar content being viewed by others
Introduction
Gene fusions constitute pivotal driver events occurring in two-thirds of mesenchymal neoplasms in children and young adults. In sarcomas, these recurrent chromosomal translocations are often the only cytogenetic abnormality and result in specific gene fusions, often encoding aberrant chimeric transcription factors. Clinically, the correlation of these translocation-derived genetic markers and discrete pathologic entities has been quite reliable, albeit, an increasing number of gene fusion events have been shown not to be histotype specific (e.g., molecular pleiotrophy).
A subset of tumors continue to defy classification based on lack of a distinctive morphology and immunohistochemical profile, necessitating the label of ‘unclassified mesenchymal neoplasms’. This category is decreasing at an accelerated pace due to the application of RNA sequencing technology on archival material. As a result, a number of new entities have recently emerged from the realm of previously unclassified spindle cell neoplasms with monomorphic cytomorphology, such as NTRK and other kinase-fusion positive spindle cell tumors [1,2,3] undifferentiated sarcomas with BCOR-CCNB3 [4, 5] and MEIS1-NCOA2 [6].
In this study we have encountered 3 low grade spindle cell sarcomas with storiform growth, uniform phenotype, and variable S100 immunopositivity, which did not fit into any well‐defined pathologic category. Targeted RNA sequencing platforms were used for further molecular characterization which uncovered recurrent novel NACC1 fusions.
Material and methods
Case selection
The cases were retrieved from the consultation files of the authors (C.R.A. and C.D.F). Three unclassified low grade spindle cell sarcomas with distinctive storiform growth and variable S100 positivity were collected. These cases were submitted for various RNA sequencing methods, two at the time of initial diagnosis for further tumor classification and one during retrospective case review for unclassified translocation-associated sarcomas. The hematoxylin and eosin stained slides and the immunohistochemical stains were reviewed. Clinicopathologic parameters, including relevant clinical history, age, gender, tumor location, tumor size, and follow-up information, were collected from the pathologic reports and electronic medical record. This study was approved by the institutional review board.
RNA sequencing
The samples were investigated on different targeted RNA sequencing platforms, including TruSight RNA Fusion Panel (Illumina, San Diego, CA) (case #1), and Archer FusionPlex Custom Solid Panel (n = 2, cases #2-3), using formalin-fixed paraffin-embedded (FFPE) tissues. The above gene panels include 507 and 85 cancer-related genes, respectively, with NACC1 gene being not included in these panels. The detailed methods have been described previously [6, 7].
Fluorescence in situ hybridization (FISH)
FISH for EWSR1, FUS and NACC1 was performed using custom designed probes made by BACs flanking respective genes as previously described [8] and listed in Supplementary Table 1 to validate the fusions. BAC clones were selected based on the information on UCSC genome browser (http://genome.ucsc.edu) and obtained from BACPAC sources of Children’s Hospital of Oakland Research Institute (CHORI) (Oakland, CA) (http://bacpac.chori.org) and Life Technologies Corporation (Carlsbad, CA). After plasmid DNA extraction and nick translation, the probes were validated on metaphases. Four µm-thick FFPE tissue sections of our cases were pretreated and hybridized with the probes. Two-color FISH was performed, first using a break-apart signal assay for each gene to document individual gene rearrangement; followed by a dual-color fusion FISH assay, showing come-together signal for validating the gene fusion event.
Results
Clinical and pathologic features
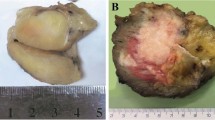
All three cases occurred in young adult female patients with a mean age of 30 years old (range 29–31 years) (Table 1). The three lesions arose in the deep soft tissues, two in the lower extremities (thigh, popliteal area) and one in the abdominal wall. Case #1 was located close to the sciatic nerve and was marginally excised to preserve the nerve. The patient received 6000 cGy external beam radiation and remained free of disease at 3.5 years follow-up. Case#2 involved the abdominal wall musculature without intra-abdominal extension and measured 15 × 12 × 7 cm grossly and appeared well-circumscribed grossly, with a pale-yellow and fleshy cut surface. The patient noted this lesion 5 years prior and it was thought to represent a lipoma. Case #3 presented with a local recurrence in the popliteal area of a lesion diagnosed a year prior.
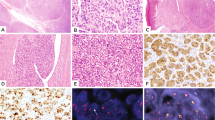
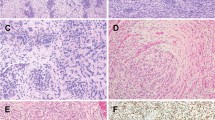
Histologically, the tumors shared a predominant storiform and pinwheel growth pattern, although 2 cases showed focal fascicular growth and solid sheet-like pattern (Figs. 1 and 2). Case #1 showed focal macro and microcystic changes containing serous fluid content (Fig. 1). Case #2 showed a partial shell of mature bone at the periphery of the lesion (Fig. 2A–G). Case # 3 showed prominent stromal vessels (Fig. 2H, I). All 3 tumors showed mainly uniform cytomorphology, with slender spindle cells having thin cell processes, scant eosinophilic cytoplasm and ovoid nuclei with vesicular or focally hyperchromatic nuclei (Figs. 1 and 2). Only scattered cells with enlarged nuclei with hyperchromasia and moderate nuclear pleomorphism were noted (Fig. 2). Mitotic figures ranged from 5-10/10 HPFs, while minute foci of necrosis were noted only in case #1. A delicate collagenous stroma was seen in all cases.
A Low power showing macro- and microcystic changes at the periphery of the lesion. B Distinctive storiform growth pattern throughout and slender cell processes. C Spindle cells with fibrillary eosinophilic cytoplasm and relatively monomorphic narrow to plump ovoid nuclei, with fine chromatin and minute nucleoli and a brisk mitotic activity (3 mitoses discerned in this 200x field). D Focal S100 protein positivity.
A Focal area of ossification present at the periphery of the lesion composed of mature lamellar bone. B The tumor showed a predominant storiform pattern, with scattered tightly packed micronodular whorls C, as well as focal fascicular growth D. D Cells showed mainly uniform cytology with open, vesicular chromatin and a brisk mitotic activity. E Areas with wiry collagen between tumor cells were also noted. F Only rare cells with mildly enlarged hyperchromatic nuclei and moderate nuclear pleomorphism. Small foci of xanthoma cells also present. G Patchy S100 positivity (red chromogen, nuclear and cytoplasmic). H Low power showing a uniformly cellular neoplasm associated with prominent small vessels. I Tumor showed a predominant storiform growth pattern composed of monomorphic spindle cells with delicate cell processes.
Immunohistochemistry
A large battery of immunohistochemical stains were performed in all 3 cases at the time of diagnosis. The only consistent immunopositivity was variable S100 protein staining which was present in all three cases (Figs. 1 and 2), while none showed SOX10, CD34, EMA, CK, TLE1, desmin, myogenin, MSA, or caldesmon positivity.
Molecular results
Case 1 was studied by TruSight RNA Fusion Panel (including a 507-gene panel) and analyzed by using both the STAR aligner and Manta fusion caller, and the BOWTIE2 aligner and JAFFA fusion caller. The analysis showed the presence of a fusion between a portion of exon 6 of FUS gene with portion of NACC1 intron 1. The other 2 cases were analyzed by Archer FusionPlex. Case 2 showed the presence of a fusion between FUS exon 6 to NACC1 exon 2, while case 3 was positive for a transcript composed of exon 7 of EWSR1 fused to exon 2 of NACC1 (Fig. 3). Based on this breakpoint, the projected fusion protein retains both the BEN and the BTB (POZ) domains of the NACC1 (nucleus accumbens-associated 1) protein (Fig. 3).
A Upper panel summarizes the chromosomal locations of EWSR1 on 22q12.2, FUS on 16p11.2 and NACC1 on 19p13.2. Red vertical lines depict the genomic break, while the orange and green arrows reveal the direction of transcription of each gene partner. B Lower panel depicts the two variant fusions, in which NACC1 exon 2 is fused either with exon 7 of EWSR1 or exon 6 of FUS. The protein domains of each gene as well as the predicted domains preserved in the fusion oncoprotein are also illustrated.
FISH analysis showed split-apart signals for individual FUS, EWSR1 and NACC1 gene break-apart assays, as well as come-together signals using the dual-color FISH assay for FUS-NACC1 fusion (Supplementary Fig. 1).
Discussion
In this study we describe 3 unique low grade spindle cell sarcomas arising in young women in deep soft tissue sharing a similar morphology of storiform growth and uniform cytology and an immunoprofile with patchy positivity for S100. Based on these findings, the tumors did not fit into any well‐defined category; being defined as unclassified sarcomas prior to molecular characterization.
The main differential diagnosis was with a low grade malignant peripheral nerve sheath tumor, due to monomorphic histology and patchy S100 positivity, which was rendered in 2 of the cases. However, none of the patients had history of NF1. Moreover, the tumors lacked evidence of a pre-existent neurofibroma, and immunophenotypically were negative for SOX10, while retaining H3K27me3 expression in all cases. The other close mimic based on their predominant storiform growth was dermatofibrosarcoma protuberans. However, tumors were all deep-seated, with no dermal component, had mostly well-circumscribed borders, and were negative for CD34. In one of the cases the possibility of a synovial sarcoma was raised due to monotonous growth, primitive cytology and fibrous stroma. However, tumors lacked reactivity for EMA, CK and TLE1, as well as SS18 gene rearrangement. Case #2 had a partial rim of ossification, raising the possibility of an ossifying fibromyxoid tumor. However, this finding was not present in the other 2 cases and no ovoid to epithelioid cells arranged in cords or nests, typically seen in ossifying fibromyxoid tumors, were noted.
EWSR1 is the prototypical ‘promiscuous gene’, with a propensity for fusing to a host of different partner genes, mostly transcription factors. It encodes the RNA-binding protein EWSR1, a member of the TET family of transcription factors, and is rearranged in a variety of mesenchymal and epithelial neoplasms. FUS, another member of the TET family, can substitute for EWSR-related fusions in the majority of tumor types [9]. Recurrent gene fusions involving EWSR1 and FUS with various transcription factors have been described in tumors of various risks of malignancy and cell lineages, including benign and malignant soft tissue tumors, carcinomas, mesotheliomas, etc. [10,11,12,13].
In contrast, no NACC1-related gene fusions have been implicated so far in human neoplasia. NACC1 (nucleus accumbens-associated 1) gene, located on 19p13.13, encodes a nuclear protein which is a member of the bric-a-brac tramtrack Broad complex/poxvirus and zinc finger (BTB/POZ) domain family, which is involved in several cellular processes including proliferation, apoptosis and transcription regulation [14]. NACC1 was originally identified and cloned as a novel transcript from the nucleus accumbens, a unique forebrain structure involved in reward motivation and addictive behaviors. The encoded protein is a transcriptional repressor that plays a role in stem cell self-renewal and maintenance of pluripotency [15, 16]. In human cancer, NACC1 is up-regulated in a variety of neoplasms, mostly carcinomas but also in uterine sarcomas [17]. The BTB/POZ gene family is composed of several proteins that share a conserved BTB/POZ protein–protein interaction motif at the N terminus that mediates homodimer or heterodimer formation [18], which is not retained in the fusion oncoprotein.
We believe the three cases in this report represent unique soft tissue tumor entity, characterized by NACC1 rearrangement. The biologic behavior of these neoplasms remains difficult to assess based on our limited sample size; however, given the potential for local recurrence we feel it prudent to consider these a low-grade sarcoma until more detailed characterization is possible.
Data availability
The Fastq data on one of the cases is available upon request.
References
Kao YC, Suurmeijer AJH, Argani P, Dickson BC, Zhang L, Sung YS, et al. Soft tissue tumors characterized by a wide spectrum of kinase fusions share a lipofibromatosis-like neural tumor pattern. Genes Chromosom. Cancer. 2020;59:575–83.
Suurmeijer AJ, Dickson BC, Swanson D, Zhang L, Sung YS, Huang HY, et al. The histologic spectrum of soft tissue spindle cell tumors with NTRK3 gene rearrangements. Genes Chromosom. Cancer. 2019;58:739–46.
Chiang S, Cotzia P, Hyman DM, Drilon A, Tap WD, Zhang L, et al. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am J Surg Pathol. 2018;42:791–8.
Argani P, Kao YC, Zhang L, Bacchi C, Matoso A, Alaggio R, et al. Primary renal sarcomas with BCOR-CCNB3 gene fusion: a report of 2 cases showing histologic overlap with clear cell sarcoma of kidney, suggesting further link between bcor-related sarcomas of the kidney and soft tissues. Am J Surg Pathol. 2017;41:1702–12.
Kao YC, Owosho AA, Sung YS, Zhang L, Fujisawa Y, Lee JC, et al. BCOR-CCNB3 fusion positive sarcomas: a clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am J Surg Pathol. 2018;42:604–15.
Argani P, Reuter VE, Kapur P, Brown JE, Sung YS, Zhang L, et al. Novel MEIS1-NCOA2 gene fusions define a distinct primitive spindle cell sarcoma of the kidney. Am J Surg Pathol. 2018;42:1562–70.
Agaram NP, Zhang L, Sung YS, Cavalcanti MS, Torrence D, Wexler L, et al. Expanding the spectrum of intraosseous rhabdomyosarcoma: correlation between 2 distinct gene fusions and phenotype. Am J Surg Pathol. 2019;43:695–702.
Antonescu CR, Zhang L, Chang NE, Pawel BR, Travis W, Katabi N, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosom. Cancer. 2010;49:1114–24.
Mertens F, Antonescu CR, Mitelman F. Gene fusions in soft tissue tumors: Recurrent and overlapping pathogenetic themes. Genes Chromosom. Cancer. 2016;55:291–310.
Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma-association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356–62.
Antonescu CR, Katabi N, Zhang L, Sung YS, Seethala RR, Jordan RC, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosom. Cancer. 2011;50:559–70.
Desmeules P, Joubert P, Zhang L, Al-Ahmadie HA, Fletcher CD, Vakiani E, et al. A subset of malignant mesotheliomas in young adults are associated with recurrent EWSR1/FUS-ATF1 fusions. Am J Surg Pathol. 2017;41:980–8.
Argani P, Harvey I, Nielsen GP, Takano A, Suurmeijer AJH, Voltaggio L, et al. EWSR1/FUS-CREB fusions define a distinctive malignant epithelioid neoplasm with predilection for mesothelial-lined cavities. Mod Pathol. 2020;33:2233–43.
Scofield MD, Korutla L, Jackson TG, Kalivas PW, Mackler SA. Nucleus Accumbens 1, a Pox virus and Zinc finger/Bric-a-brac Tramtrack Broad protein binds to TAR DNA-binding protein 43 and has a potential role in Amyotrophic Lateral Sclerosis. Neuroscience. 2012;227:44–54.
Nakayama K, Nakayama N, Wang TL, Shih IM. NAC-1 controls cell growth and survival by repressing transcription of Gadd45GIP1, a candidate tumor suppressor. Cancer Res. 2007;67:8058–64.
Nakayama N, Kato H, Sakashita G, Nariai Y, Nakayama K, Kyo S, et al. Protein complex formation and intranuclear dynamics of NAC1 in cancer cells. Arch Biochem Biophys. 2016;606:10–5.
Rahman MT, Nakayama K, Ishikawa M, Rahman M, Katagiri H, Katagiri A, et al. NAC1, a BTB/POZ protein overexpressed in uterine sarcomas. Anticancer Res. 2012;32:3841–5.
Nakayama K, Nakayama N, Davidson B, Sheu JJ, Jinawath N, Santillan A, et al. A BTB/POZ protein, NAC-1, is related to tumor recurrence and is essential for tumor growth and survival. Proc Natl Acad Sci USA. 2006;103:18739–44.
Acknowledgements
We would like to thank Dr. Annabelle Mahar, Royal Prince Alfred Hospital, Camperdown, NSW, Australia and Dr. Elias Nasser, Illawarra Cancer Care Center, Woolongong, NSW, Australia, for providing clinical information and follow-up. Also, we would like to thank Bruce Crilly for his excellent assistance with the graphic design for the image composites and Milagros Soto for editorial assistance.
Funding
Supported by: P50 CA 140146-01 (CRA), P30 CA008748 (CRA), Cycle for Survival (CRA), St Baldrick Foundation (CRA), Kristin Ann Carr Foundation (CRA).
Author information
Authors and Affiliations
Contributions
CRA, BD and CDF participated in the concept of the study, CRA wrote the manuscript, LZ performed the FISH experiments, YSS performed the bioinformatic analysis and fusion diagrams; all authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study was approved at the participating institutions IRB.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Antonescu, C.R., Dickson, B.C., Zhang, L. et al. Unclassified low grade spindle cell sarcoma with storiform pattern characterized by recurrent novel EWSR1/FUS-NACC1 fusions. Mod Pathol 34, 1541–1546 (2021). https://doi.org/10.1038/s41379-021-00805-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-021-00805-x
This article is cited by
-
EWSR1-induced circNEIL3 promotes glioma progression and exosome-mediated macrophage immunosuppressive polarization via stabilizing IGF2BP3
Molecular Cancer (2022)
-
A Low Grade Nasopharyngeal sarcoma With FUS::NACC1 Fusion and Immunohistochemical Evidence of Epithelial Differentiation: Expanding the Clinicopathologic Spectrum of an Emerging Entity
Head and Neck Pathology (2022)