Abstract
Mucinous ovarian tumors rarely harbor mural nodules, which have historically been classified as sarcoma-like, anaplastic carcinomatous, or sarcomatous on the basis of predominant morphologic features. The molecular relationship between mural nodules and associated mucinous ovarian tumors remains poorly characterized, as does the molecular pathogenesis of these mural nodules. Thus, we analyzed the morphological, immunohistochemical, and genetic features of 13 mucinous ovarian tumors and associated mural nodule(s). Three harbored sarcoma-like mural nodules and ten contained anaplastic carcinomatous nodules, including 1 tumor with spatially discrete anaplastic carcinomatous and sarcomatous nodules. Twelve of 13 cases showed genetic evidence of clonality between the mural nodule(s) and associated mucinous ovarian tumor, including all three tumors with sarcoma-like morphology. Mural nodules were genetically identical in the five cases in which there were multiple discrete mural nodules that were sequenced separately. MTAP and p53 immunohistochemistry confirmed the distribution of neoplastic cells in a subset of sarcoma-like and anaplastic carcinomatous nodules. No single recurrent genetic alteration was associated with mural nodule development. No recurrent genetic differences were identified between mural nodules with sarcoma-like, anaplastic carcinomatous, and sarcomatous morphology. Of 11 patients with clinical follow-up, three died of disease 3, 8, and 9 months after diagnosis, but no recurrent genetic events were associated with poor outcome. These molecular data suggest that sarcoma-like, anaplastic carcinomatous, and sarcomatous nodules represent a morphologic spectrum of clonal neoplasms arising in mucinous ovarian tumors rather than three discrete biological entities.
Similar content being viewed by others
Introduction
Rarely, mucinous ovarian tumors—predominantly borderline tumors and differentiated (gland-forming) adenocarcinomas—harbor one or more mural nodules, which have historically been classified into three morphologic categories: sarcoma-like mural nodules, anaplastic carcinomatous nodules, and sarcomatous nodules [1,2,3,4,5]. Sarcoma-like mural nodules have been generally regarded as benign reactive lesions, whereas anaplastic carcinomatous and sarcomatous nodules are considered to be aggressive malignant neoplasms, giving great clinical importance to the distinction of sarcoma-like nodules from anaplastic carcinomatous and sarcomatous nodules [1, 6]. Morphologically, sarcoma-like nodules are described as sharply circumscribed, often protruding into the lumen of a mucinous cyst, and composed of a mixed cell population, including prominent inflammatory cells and osteoclast-like giant cells, which may be most prominent around a central hemorrhagic cavity [1, 4]. In contrast, anaplastic carcinomatous nodules and sarcomatous nodules are described as poorly circumscribed infiltrative lesions with or without lymphovascular invasion, generally dominated by a mono- or multinucleated malignant tumor cells, with less prominent inflammatory infiltrate [2, 3, 5]. Despite the described morphologic distinctions, accumulated experience has shown that these three subtypes of mural nodules are not always easily distinguished morphologically. Necrosis, marked nuclear atypia, and abundant mitoses (including atypical forms) have been accepted in sarcoma-like nodules, as well as in anaplastic carcinomatous and sarcomatous nodules [4, 5].
The most common molecular alterations in mucinous adenocarcinomas of the ovary include mutations or copy number alterations in CDKN2A and mutations in TP53 and KRAS. Recurrent amplification of ERBB2 and mutations in BRAF, PIK3CA, and ARID1A are also reported [7, 8]. However, little is known about the molecular features of mural nodules arising in mucinous ovarian tumors. A 2015 case report identified a shared KRAS p.G12D mutation in an anaplastic carcinomatous nodule and associated well-differentiated mucinous adenocarcinoma, providing the first molecular evidence that mural nodules can be clonally derived from associated mucinous ovarian tumors [9]. A subsequent study of seven anaplastic carcinomatous nodules and paired mucinous ovarian tumors likewise found evidence of clonality in all pairs, with frequent mutations in KRAS, TP53, PTEN, and PIK3CA [10]. However, the number of reported sequenced mural nodules remains in single digits, and only anaplastic carcinomatous-type nodules have been sequenced. Therefore, we conducted a study to molecularly assess and compare the different classifications of mural nodules arising in mucinous ovarian tumors.
Methods
Cohort selection
Following institutional review board approval, an electronic search of the archives of the Division of Women’s and Perinatal Pathology identified 31 mucinous ovarian tumors with mural nodules. Formalin-fixed paraffin-embedded tissue sufficient for next-generation sequencing was available for 13 tumors from 13 patients, diagnosed between 1996 and 2019: nine in-house cases and four personal consults to two of the authors (CPC, MRN). Medical records were reviewed for patient age, surgical procedure, adjuvant therapy, tumor stage (FIGO 2014) and laterality, disease-free survival, and disease-specific survival. Pathology reports were reviewed for tumor stage and laterality, gross size and description of the mucinous ovarian tumor and mural nodule(s), and number of mural nodules per tumor.
Morphologic review
All available hematoxylin and eosin-stained tumor slides were reviewed for each case (range 1–38; mean 12; median 5). The total number of tumor blocks submitted ranged from 7 to 36 (mean 17; median 16) per case, equating to 0.5–2.0 (mean 1.0; median 0.8) blocks per cm of tumor. In each case, the background mucinous tumor was subtyped morphologically, and each mural nodule was evaluated for microscopic circumscription; nuclear atypia; mitotic rate; inflammatory infiltrate; and presence of atypical mitoses, necrosis, lymphovascular invasion, rhabdoid cells, osteoclast-like giant cells, and central hemorrhagic cavitation. Morphologic details in pathology reports were also reviewed. Each mural nodule was classified as sarcoma-like, anaplastic carcinomatous, or sarcomatous on the basis of published morphologic descriptions [1,2,3,4,5]. Cases with classification complicated by mixed or ambiguous morphologic features were noted.
Immunohistochemistry
In cases with sufficient available tissue, the mural nodule(s) and associated mucinous ovarian tumor were immunostained (4-µm sections) for cytokeratin (AE1/AE3, Dako, 1:200), p53 (clone DO-7, Dako, 1:500), INI1 (clone 25, BD Bioscience, 1:600), MTAP (clone 42-T, Santa Cruz, 1:75), and HER2 (clone SP3, Cell Marque, 1:75). Immunohistochemistry results from pathology reports were also reviewed.
Cytokeratin immunoexpression was compared with the morphologic classification of each mural nodule. More than patchy cytokeratin expression prompted re-evaluation of a sarcoma-like mural nodule; any cytokeratin expression prompted re-evaluation of a sarcomatous nodule; and absence of cytokeratin expression prompted re-evaluation of an anaplastic carcinomatous nodule. These considerations are in keeping with previous publications [4, 5].
P53 immunostains were interpreted as strong-diffuse mutant pattern if strong nuclear staining was seen in >80% of tumor cells; as null-mutant pattern if nuclear staining was completely absent; as cytoplasmic mutant if there was unequivocal cytoplasmic and variable nuclear staining; and as wild type if none of these mutant patterns was present. MTAP immunostains were interpreted as lost in the complete absence of tumor cell cytoplasmic staining. INI1 immunostains were interpreted as lost in the absence of tumor cell nuclear staining. Non-tumor cells provided a positive internal control in p53, MTAP, and INI1 immunostains. HER2 immunostains were interpreted according to 2018 guidelines from the American Society of Clinical Oncologists and College of American Pathologists [11].
Next-generation sequencing
In each case, formalin-fixed paraffin-embedded tissue from the mural nodule(s) and from the background mucinous ovarian tumor was separately dissected and sequenced on the 447-gene OncoPanel next-generation sequencing platform [12]. Each case was annotated for point mutations, small insertions and deletions (indels), copy number alterations, microsatellite instability, and tumor mutational burden. Two grossly or microscopically discrete mural nodules were separately sequenced in five cases, and a tumor component of well-differentiated endometrioid carcinoma was separately sequenced in 1 case (#10).
All molecular variants were filtered to remove technical artifacts, synonymous variants, and any population variants at greater than 0.1% frequency in the Genome Aggregation Database (https://gnomad.broadinstitute.org/). The remaining pass-filter variants were assessed for likely pathogenecity by a molecular pathologist (LMS). Mutational profiles for each mucinous ovarian tumor and paired mural nodule(s) were compared. Presence of at least one shared pathogenic mutation was considered evidence of clonality. Specimens with greater than 3 copy number alterations were considered to have complex copy number alterations. Microsatellite stability status and tumor mutational burden were determined as previously described [13, 14].
Statistical analyses were performed with SAS v9.4 (SAS Institute; Cary, NC).
Results
Cohort characteristics
Clinical parameters are summarized in Table 1. The study cohort included 12 women with mural nodules arising in mucinous ovarian tumors and 1 woman with an anaplastic carcinomatous nodule arising in a primary retroperitoneal multicystic mucinous tumor. Presence of a mural nodule was not diagnosed preoperatively in any case. Patients ranged from 16 to 77 years old at time of surgery (median 45; mean 44). Three patients underwent salpingo-oophorectomy only, three underwent salpingo-oophorectomy with staging, and six underwent hysterectomy with bilateral salpingo-oophorectomy and staging. The patient with the primary retroperitoneal mucinous tumor underwent local excision only. Six mucinous ovarian tumors were primary in the right ovary and in six in the left ovary. Seven cases were stage IA, 1 IC1, 1 IC2, 1 II, 1 IIIA, and 2 IIIB. Lymph nodes were sampled in four patients, with no lymph node metastases identified.
Gross and microscopic findings
Gross and microscopic findings are detailed in Table 2. Tumors ranged from 5 to 40 cm (mean 21; median 25). Ten tumors were grossly described as multiloculated cysts and three as unilocular. A mural nodule was grossly identified in eight cases, and multiple (range, 2–10) mural nodules were seen on gross examination in 5. On microscopic examination, multiple discrete mural nodules were identified in one additional case. The mural nodules ranged from 0.5 to 9.0 cm (mean 3.2; median 1.8).
A sarcoma-like mural nodule was identified in three cases, including one with multiple mural nodules showing similar sarcoma-like morphology. An anaplastic carcinomatous nodule was identified in ten cases, including three with multiple mural nodules showing the same anaplastic carcinomatous morphology and one with grossly and microscopically discrete anaplastic carcinomatous and sarcomatous nodules. No case contained a sarcomatous nodule only, and no teratomatous elements were present in any case.
Anaplastic carcinomatous nodules were more often diagnosed in older women (range, 26–71 years) and were often larger (range, 0.5–9 cm), compared with sarcoma-like mural nodules (age range, 16–41 years; size range, 0.8–5.6 cm). However, these size and age distributions were not significantly different (P = 0.70 and 0.06, respectively; two-tailed t test), in keeping with prior reports [4, 5].
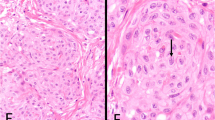
All three sarcoma-like mural nodules were well-circumscribed and showed the “pleomorphic and epulis-like” morphology originally described by Prat and Scully (Fig. 1) [1]. Three were lined at least focally by denuded mucinous epithelium, often most prominent at the shoulders of an exophytic sarcoma-like mural nodule. Aneurysmal bone cyst-like distribution of osteoclast-like giant cells around a central hemorrhagic cyst was seen in sarcoma-like mural nodules only (see Fig. 1d).
A sarcoma-like mural nodule (Case #11) is well-circumscribed (a, ×20), with a central hemorrhagic cyst (b, ×20) showing an aneurysmal bone cyst-like arrangement of osteoclast-like giant cells (c, ×100) and a mixed cell population (d, ×200). A second sarcoma-like mural nodule (#13) is well-circumscribed and protrudes into a cystic lumen (e, ×4). The shoulder of the nodule is lined by mucinous epithelium (f, ×100). The mixed cell population (g, ×200) shows strong-diffuse mutant-pattern p53 expression (h, ×200, p53 IHC) and rare cytokeratin-positive cells (i, ×200, AE1/AE3 IHC).
Of eight anaplastic carcinomatous nodules, seven showed pleomorphic (mixed epithelioid and sarcomatoid), two showed epithelioid, and one showed sarcomatoid morphology, as described by Provenza et al. (Fig. 2) [5]. Eight showed an infiltrative tumor-stromal interface, whereas two were well-circumscribed with an expansile growth pattern. In four cases, the anaplastic carcinomatous component merged microscopically with foci of infiltrative gland-forming mucinous adenocarcinoma. All three stage III cases showed anaplastic carcinomatous nodules in the tumor primary site, and all metastases showed only anaplastic carcinoma-type morphology (i.e., no gland-forming adenocarcinoma component present).
A anaplastic carcinomatous nodule (Case #3) shows mixed epithelioid and sarcomatoid (pleomorphic) morphology (a, ×200), patchy cytokeratin expression (b, ×200, AE1/AE3 IHC), and strong-diffuse mutant-pattern p53 expression (c, ×200, p53 IHC). Metaplastic bone formation is also seen (d, ×100). A second anaplastic carcinomatous nodule (Case #5) is well-circumscribed (e, ×40) and shows epithelioid morphology with only modest nuclear atypia and a brisk inflammatory infiltrate (f, ×200), strong-diffuse mutant-pattern p53 expression (g, ×200, p53 IHC) and MTAP loss (h, ×200, MTAP IHC). Strong membranous HER2 expression (i, ×200, HER2 IHC) is seen in the epithelium of the adjacent mucinous borderline tumor (left) but not in the anaplastic carcinomatous nodule (right). In one focus (j, ×100), the anaplastic carcinomatous component (right) merges with better differentiated gland-forming mucinous adenocarcinoma.
The sole sarcomatous nodule was well-circumscribed and comprised slender spindle cells with herringbone architecture (Fig. 3). As noted above, this sarcomatous nodule was present in a mucinous ovarian tumor that also harbored a second discrete pleomorphic anaplastic carcinomatous nodule.
The sarcomatous nodule is well-circumscribed (a, ×40) and shows a herringbone arrangement and brisk mitotic activity (b, ×200). The separate anaplastic carcinomatous nodule shows predominantly epithelioid morphology with marked atypia and brisk mitoses (c, ×200). These two nodules were genetically identical.
This cohort is too small for adequately powered statistical comparisons of individual morphologic features, and P values were not significant for differences in associated mucinous ovarian tumor histotype, or for mural nodule circumscription, rhabdoid cells, osteoclast-like giant cells, central cavitation and hemorrhage, necrosis, mitotic rate, or atypical mitoses.
Three mural nodules were difficult to classify morphologically:
-
The mural nodule in Case 1 showed an infiltrative border and was classified as an anaplastic carcinomatous nodule, but also showed some sarcoma-like features, including prominent hemorrhage and osteoclast-type giant cells.
-
The mural nodule in Case 11 had a large central hemorrhagic cavity and abundant osteoclast-like giant cells, and was classified as a sarcoma-like mural nodule, but also showed a slightly irregular periphery and a more monomorphous cell population focally, resembling an anaplastic carcinomatous nodule. Case 11 showed null-mutant p53 immunostaining in both the sarcoma-like and anaplastic carcinoma-like foci, indicating that both components are a single clonal population.
-
The mural nodule in Case 7 showed an overtly infiltrative border and was classified as an anaplastic carcinomatous nodule. However, it also showed a mixed cell population with marked inflammatory infiltrate (including prominent osteoclast-like giant cells) and central hemorrhagic cavitation, suggesting superimposed sarcoma-like features.
Three cases showed additional unusual morphologic features:
-
One case (#10) showed four spatially discrete morphologic components: a conventional mucinous borderline tumor, a well-differentiated endometrioid carcinoma (without mucinous features), an anaplastic carcinomatous nodule with pleomorphic (mixed epithelioid and spindled) pattern, and a sarcomatous nodule with herringbone architecture (see Supplemental Fig. 1). All four components were genetically identical (see below).
-
One stage IIIA anaplastic carcinomatous nodule (Case 2) manifested with scattered, microscopic clusters of tumor cells set in multifocal, grossly evident patches of intra-abdominal mucin. The associated mucinous borderline tumor was positive for CK7 and negative for CK20, and showed no morphologic features suggesting metastasis from a gastrointestinal primary [15]. The appendix was clinically and histologically unremarkable, and the patient was free of disease 20 months after diagnosis.
-
Metaplastic bone formation was seen in one anaplastic carcinomatous nodule (Case 3) (see Fig. 2d).
Molecular characterization
Molecular results are summarized in Fig. 4 (see also Supplemental Data). Molecular evidence for clonality between the background mucinous ovarian tumor and associated mural nodule(s) was found in 12 of 13 cases (92%). Multiple, separately sequenced mural nodules from the same tumor were molecularly identical in five of five cases. In a single case, a discrete component of well-differentiated endometrioid adenocarcinoma was likewise clonally related to the associated mucinous borderline tumor, anaplastic carcinomatous nodule, and sarcomatous nodule. The single non-clonal pair (Case 8) included a stage IIIB pleomorphic anaplastic carcinomatous nodule associated with a mucinous borderline tumor.
AC anaplastic carcinoma nodule, ACA adenocarcinoma, DOC dead of other causes, DOD dead of disease, Epi epithelioid pattern, Exp expansile carcinoma, IEC with intraepithelial carcinoma, Inf infiltrative carcinoma, MI with microinvasion, MOT mucinous ovarian tumor, MN mural nodule, MSS microsatellite stable, Mutant strong-diffuse mutant, NED no evidence of disease, Null null-mutant, P pleomorphic (spindled and epithelioid) pattern, PE pleomorphic and epulis-like pattern, SL sarcoma-like mural nodule, SN sarcomatous nodule, S sarcomatoid, w.t. wild type.
Shared pathogenic KRAS alterations were detected in the mucinous ovarian tumor and associated mural nodule(s) in 10 of 13 cases, including a shared missense mutation in 9 and shared amplification in 3. Three cases harbored KRAS amplification in only one component. Of four tumors without KRAS missense mutations, two harbored other RAS pathway alterations, including shared MAP2K1 missense mutation in one case and shared NRAS and BRAF mutations in one case. The other two cases each harbored a pathogenic PIK3CA missense mutation. In one case, the PIK3CA mutation was shared by mucinous borderline tumor, well-differentiated endometrioid carcinoma, anaplastic carcinomatous nodule, and sarcomatous nodule components, but in the other case (#8) the PIK3CA mutation was only in the mucinous borderline tumor, which shared no molecular alterations with its paired anaplastic carcinomatous nodule.
Ten cases harbored pathogenic TP53 alterations, including shared alterations in the mucinous ovarian tumor and associated mural nodule(s) in eight cases, three of which also showed shared 17p deletion, consistent with biallelic TP53 loss. In one case (#13), the mucinous adenocarcinoma and paired sarcoma-like mural nodule showed different TP53 mutations. One case (#3) had a missense mutation in the anaplastic carcinomatous nodule only.
Eight cases harbored pathogenic CDKN2A alterations, including shared alterations in the mucinous ovarian tumor and associated mural nodule(s) in seven cases. One case showed CDKN2A deletion in the anaplastic carcinomatous nodule only. MTAP was co-deleted with CDKN2A in four of six cases, and CDKN2B deletion was seen in eight tumors.
Four cases showed ERBB2 amplification, which was confined to the mucinous ovarian tumor in three cases and shared by mucinous adenocarcinoma and anaplastic carcinomatous nodule in one case. A nonsense mutation in SMARCB1 was confined to the anaplastic carcinomatous nodules of one case. One case each showed point mutations in KMT2A, ARID1A, and FBXW7.
Eight cases showed complex copy number alterations, which were shared between the mucinous ovarian tumor and associated mural nodule(s) in five cases, and present only in the mural nodule in three.
All mucinous ovarian tumors and mural nodules were microsatellite stable. Tumor mutational burden ranged from 2.3 to 12.9 mutations/megabase (median 5.3, mean 5.0) in mucinous ovarian tumors and from 2.3 to 13.7 mutations/megabase (median 3.8, mean 4.7) in mural nodules (P = 0.41; paired t test).
Immunohistochemistry
Immunohistochemical findings are summarized in Fig. 4. Of three sarcoma-like mural nodules, one showed patchy cytokeratin expression and two showed rare cytokeratin-positive cells (see Fig. 1i). Of ten anaplastic carcinomatous nodules, six showed diffuse cytokeratin expression, three showed patchy cytokeratin expression, and one showed a rare focus of cytokeratin-positive cells (see Fig. 2b). In one case (#9) with two grossly discrete but morphologically identical anaplastic carcinomatous nodules, one nodule showed patchy cytokeratin expression, whereas the second nodule was negative for cytokeratin. The single sarcomatous nodule (#10) was negative for cytokeratin expression. Three mural nodules that were morphologically indeterminate for anaplastic carcinomatous versus sarcomatous nodule (#8, 9, and 10) were classified as anaplastic carcinomatous nodules due to positive cytokeratin expression.
P53 immunohistochemistry was performed in ten cases. Of five cases with a TP53 missense mutation, strong-diffuse mutant-pattern p53 expression was seen in two (see Fig. 2c, g) and wild-type pattern in two, whereas one case (#10) showed strong-diffuse mutant-pattern staining in the mural nodules but wild-type staining in the well-differentiated mucinous and endometrioid components (see Supplemental Fig. 2). Strong-diffuse mutant-pattern expression was also seen in one case (#5) with a TP53 indel. Null-mutant p53 immunophenotype was seen in two cases: one with shared TP53 frameshift and 17p deletion in a mucinous borderline tumor and sarcoma-like mural nodules, and one with shared TP53 nonsense mutation and 17p deletion in a mucinous adenocarcinoma and anaplastic carcinomatous nodules. Two cases with no TP53 alteration showed wild-type immunophenotype. In cases with mutant-pattern (diffuse or null) p53 staining, admixed multinucleated osteoclast-like giant cells consistently showed wild-type p53 expression.
In this small cohort, MTAP loss by immunohistochemistry was sensitive and specific for MTAP deletion (see Fig. 2h), and was a specific marker of CDKN2A deletion. Both cases with CDKN2A deletion and normal MTAP copy number had insufficient material for immunohistochemistry.
Retained INI1 immunostaining was seen in ten of ten cases without SMARCB1 point mutation (including in three cases with SMARCB1 deletion). The single case with SMARCB1 nonsense mutation had insufficient tissue for immunohistochemistry.
In three cases with ERBB2 amplification in the background mucinous ovarian tumor only, HER2 immunohistochemistry was positive only in the mucinous ovarian tumor (see Fig. 2i). The one case with ERBB2 amplification in both the mucinous adenocarcinoma and anaplastic carcinomatous components had insufficient tissue for immunohistochemistry.
Outcomes
Clinical follow-up was available for 11 patients. Over a period of 3–279 months (median 11; mean 54), seven patients had no evidence of disease, one had died of other causes, and three died of disease at 3, 8, and 9 months. All patients with available follow-up received adjuvant chemotherapy.
-
One fatal case (#4) comprised a mucinous borderline tumor and clonally related stage IA pleomorphic anaplastic carcinomatous nodules with a shared MAP2K1 missense mutation, as well as a SMARCB1 nonsense mutation unique to the anaplastic carcinomatous nodule. The patient recurred after 4 months and died of disease 9 months after surgery.
-
A second fatal case (#8) comprised a mucinous borderline tumor with PIK3CA missense and KMT2A nonsense mutations and a non-clonal stage IIIB pleomorphic anaplastic carcinomatous nodule with complex copy number alterations. The patient died of disease 8 months after surgery.
-
The third fatal case (#9) comprised a mucinous adenocarcinoma and clonally related stage IIIB anaplastic carcinomatous nodules, with shared NRAS, BRAF, TP53, and CDKN2A mutations; shared 17p deletion; and complex copy number alterations found only in the anaplastic carcinomatous nodules. The patient recurred after 1 month and died of disease 3 months after surgery.
Although this cohort is too small for adequately powered statistical comparisons of individual morphologic or molecular parameters with patient outcome, some observations warrant mention. First, all three patients who died of disease lacked KRAS alterations, whereas none of the six patients with KRAS alterations and clinical follow-up had died of disease. Second, all three patients who died of disease had anaplastic carcinomatous nodules, whereas both patients with a sarcoma-like mural nodule and clinical follow-up were disease-free. Third, the single patient (#9) with lymphovascular invasion died of disease. P values (log-rank test) were not significant for association of disease-specific survival with background mucinous ovarian tumor histotype; with number of mural nodules; with mural nodule circumscription, mitotic index, necrosis, central cavitation, rhabdoid cells, or inflammatory infiltrate; or with TP53 alteration, CDKN2A alteration, 17p deletion, or complex copy number alteration.
Discussion
This is the largest molecular-based study to date of mucinous ovarian tumors with mural nodules; the first comprehensive comparison of sarcoma-like mural nodules, anaplastic carcinomatous nodules, and sarcomatous nodules; and the first comprehensive assessment of multiple mural nodules arising in a single tumor. Table 3 summarizes the literature published on this subject. Our data offer three principal insights into the biology of these lesions. First, there is a clonal relationship between a large majority of mural nodules and associated mucinous ovarian tumors, including in sarcoma-like mural nodules. Second, mural nodules show certain recurrent genetic alterations, but we identify no clear single genetic “trigger” for development of a mural nodule within a mucinous ovarian tumor. Third, morphologically defined sarcoma-like mural nodules, anaplastic carcinomatous nodules, and sarcomatous nodules do not show clear reproducible genetic differences, suggesting that they represent a continuum of neoplastic outgrowths in mucinous ovarian tumors, rather than three biologically discrete entities.
Historically, the three types of mural nodules were postulated to constitute two biological and clinical groups: whereas anaplastic carcinomatous and sarcomatous nodules were presumed (and, for anaplastic carcinomatous nodules, shown [9, 10]) to represent potentially aggressive clonal derivatives of the associated mucinous neoplasms, sarcoma-like mural nodules were thought to be benign reactive processes [1, 6, 16, 17]. Our data provide the first molecular evidence that all types of mural nodules, including those with classical sarcoma-like morphology, represent clonal derivatives of their associated mucinous ovarian tumors in at least a substantial proportion of cases.
Furthermore, we employed P53 and MTAP immunohistochemistry in two sarcoma-like mural nodules to identify the tumor cell population harboring TP53 and MTAP alterations with the reactive inflammatory background. This immunohistochemical–molecular correlation validates the earlier suggestion, based on morphology and p53 immunohistochemistry, that at least some sarcoma-like mural nodules include a population of entrapped tumor cells within a predominantly inflammatory background [3, 5, 18]. In contrast, our immunohistochemical demonstration of intact tumor cells refutes an earlier suggestion that sarcoma-like mural nodules may constitute reactive histiocytic lesions that phagocytose denuded tumor cells [1, 4]. In this phagocytic model, a purely sequencing-based study (without immunohistochemical correlation) could misinterpret tumor DNA from phagocytosed displaced tumor cells as evidence for clonality (although lysosomal degradation of nucleic acids might make this less likely). Finally, consistently wild-type p53 immunostaining in the osteoclast-like giant cells in both sarcoma-like and anaplastic carcinomatous nodules is in keeping with previous comparisons with aneurysmal bone cyst [1, 4].
Although our data show that most mural nodules are clonally derived from better differentiated mucinous ovarian tumors, our cohort also includes one case without evidence for clonality. Although very early phylogenetic divergence cannot be entirely excluded, we interpret this case as a collision of a mucinous borderline tumor and an anaplastic sarcomatoid carcinoma, given that the latter shows patchy cytokeratin expression and harbors complex copy number alterations, but lacks the genetic hallmarks of ovarian mucinous neoplasia seen in the other 12 cases [7, 8]. The dismal outcome in this case may suggest that these rare collisions tumors carry high risk for aggressive behavior, although molecular characterization of additional cases is needed. However, the anaplastic mural nodule in our collision tumor did not show morphological or immunophenotypic features appreciably different from the 12 clonal cases, suggesting that prospective identification of these rare collision tumors may prove difficult in clinical practice without routine application of molecular methods.
Our data did not identify any single recurrent genetic alteration as a “trigger” for the development of mural nodules in mucinous ovarian tumors. In fact, the mural nodules in most cases showed no novel genetic alterations, compared with the background mucinous ovarian tumor. The only previous molecular-based series of such tumors identified TP53 mutations in a subset of anaplastic carcinomatous nodules but not in paired mucinous ovarian tumors, prompting the authors to suggest that TP53 mutation may trigger mural nodule development [10]. However, in our study, only 1 of 13 cases showed a TP53 mutation in the mural nodule but not the associated differentiated mucinous tumor, and in 8 of 13 cases both tumor components harbored the same TP53 mutation. These data argue quite convincingly that TP53 mutation is not the key precipitant of mural nodule development. The same study also identified a CDH1 mutation in one case [10], but the pathogenicity of that mutation is in doubt, and we identified no CDH1 alterations in our 13 cases.
The mucinous ovarian tumors sequenced in this study did not show ascertainable genetic differences from large cohorts of mucinous ovarian tumors published in the literature, with similarly high rates of alterations in CDKN2A, KRAS, and TP53, as well as less frequent alterations in ERBB2, BRAF, and PIK3CA [7, 8]. Thus, we found no evidence that the genetic makeup of particular mucinous ovarian tumors may predict a predisposition to mural nodule development.
Recent work identified loss of one or more of the SWI/SNF chromatin remodeling proteins SMARCA4, SMARCB1, ARID1A, and ARID1B in 33/40 dedifferentiated endometrial endometrioid and 3/3 dedifferentiated ovarian endometrioid carcinomas, suggesting that SWI/SNF alterations could play an important role in dedifferentiation of gynecologic tumors [19, 20]. In keeping with this, we identified a non-shared deleterious SMARCB1 mutation in one anaplastic carcinomatous nodule with a fatal outcome, as well as a non-shared ARID1A frameshift mutation in one sarcoma-like mural nodule without available follow-up information. These findings suggest that SWI/SNF alterations may play a pathogenetic role in a subset of mural nodules, and may be associated with aggressive clinical behavior in some cases. Our molecular data build on a recent immunohistochemistry-based study, which reported loss of one or more SWI/SNF proteins in 9 of 25 mural nodules, with retained SWI/SNF expression in all associated mucinous ovarian tumors, and a trend toward poorer clinical outcomes in patients with SWI/SNF alterations [21]. While the specific prognostic significance of SMARCB1 loss was not discussed in that study, the single patient with SMARCB1 loss and clinical follow-up had died of disease four months after diagnosis [21]. In addition, one recent case of a young woman with rapidly progressive stage IA anaplastic carcinomatous nodule with rhabdoid morphology is suspect for a SWI/SNF alteration, but immunohistochemical and molecular testing was not reported [22]. Additional studies are indicated to better characterize the pathogenetic role of SWI/SNF alterations in mural nodules arising in mucinous ovarian tumors, as well as the prognostic value of SWI/SNF immunohistochemistry, particularly in stage IA tumors.
Increased copy number alterations were also associated with pathogenic progression in mucinous ovarian tumors in one recent large study [9]. In the current study, the greater prevalence of complex copy number alterations in mural nodules than in background mucinous ovarian tumors may suggest that these changes play a role in mural nodule development. However, complex copy number alterations were neither universal in nor specific to mural nodules in this study, nor were they associated with clinical outcome, and their pathogenetic role in progression of mucinous ovarian tumors remains unclear.
The precise role of KRAS mutation in mural nodule pathogenesis and behavior is also unclear. KRAS mutations were identified in all nine mucinous ovarian tumors with mural nodules sequenced in earlier studies [9, 10, 23], but we report three tumors with no KRAS mutation in either component, suggesting that KRAS mutations may not be significantly more prevalent in mucinous ovarian tumors with mural nodules than in those without, as previously suggested [10, 24]. Interestingly, in our cohort, absence of a KRAS alteration was highly correlated with disease-specific mortality. However, disease-related deaths have been reported in patients with KRAS-mutated mural nodules, indicating that KRAS mutation is not invariably associated with improved outcome [10].
Histomorphologic findings are prognostic in a subset of cases. Extrapelvic dissemination was associated with death from disease in two of three cases, in keeping with prior work [5] and highlighting the importance of careful examination of the abdominal cavity during surgery for otherwise unremarkable ovarian mucinous lesions, as the mural nodule was identified only on pathologic examination in all 13 of our cases. Lymphovascular invasion also correlated with a poor outcome. However, we found that some otherwise characteristic anaplastic carcinomatous nodules may be well-circumscribed (although only select slides were reviewed in one such case), including in an exemplary stage IA, SMARCB1-mutated anaplastic carcinomatous nodule in a 33-year-old woman which resulted in death from disease 9 months after diagnosis. Thus, neither low tumor stage nor sharp circumscription nor young age guarantees a benign clinical course. Furthermore, in keeping with prior series, our data show that death from disease is typically an early outcome, occurring within 1 year of diagnosis [3, 5].
The limited immunopanel in this study served primarily to define the distribution of neoplastic cells in tumor sections. However, we also found no association between patient outcome and mutant-pattern p53 expression, MTAP loss, or CDKN2A alterations in this small cohort. P53 immunohistochemistry and TP53 mutational status were discordant in some cases, wild-type-pattern p53 immunostaining seen in three tumors with TP53 missense mutations. A recent abstract [25] reported modified criteria for interpreting p53 immunohistochemistry in mucinous ovarian tumors, which yielded strong correlation between immunohistochemistry and TP53 molecular sequencing. However, that work has not yet been reported in a detailed manuscript, and careful review of our discordant cases revealed conventional wild-type p53 staining (see Supplemental Fig. 2).
The absence in this study of recurrent genetic differences between sarcoma-like, anaplastic carcinomatous, and sarcomatous mural nodules is in keeping with longstanding documentation of substantial morphological overlap between sarcoma-like, anaplastic carcinomatous, and sarcomatous nodules.
-
First, a given mural nodule may be difficult to precisely classify as one of these three types. As previously reported and noted in three cases in this study, a mural nodule may show overlapping sarcoma-like and anaplastic carcinomatous features [4, 5, 17, 22, 26]. Rare sarcoma-like nodules may also mimic osteosarcoma or other mesenchymal malignancy [26,27,28,29]. Given the benign nature previously attributed to sarcoma-like nodules, these distinctions would be of clinical importance. Although potentially less clinically significant, anaplastic carcinomatous and sarcomatous nodules also show substantial morphologic overlap (as in Cases 8, 9, and 10 in this study), and objective criteria for reproducibly distinguishing these subtypes are lacking [30, 31].
-
Second, multiple discrete nodules within a single tumor may show mixed morphologic patterns [4, 26, 32]. In Case 10 in this study, physically discrete but molecularly identical mural nodules showed unequivocally distinct carcinomatous and sarcomatous morphologies.
Morphologic, immunophenotypic, and molecular overlap suggests that mural nodules represent a continuum of clonal neoplasia arising in mucinous ovarian tumors, rather than multiple biologically distinct entities. However, the two patients in this study with sarcoma-like mural nodules and clinical follow-up had a favorable outcome, and demonstration of clonality does not inherently dispute previously published evidence that rigorously defined sarcoma-like nodules are not associated with increased patient risk. Although we did not identify a genetic cause for the clinical differences between sarcoma-like and anaplastic carcinomatous nodules, subsequent studies may identify epigenetic or other factors to account for this difference. For now, though, it remains reasonable (among stage IA mural nodules) to distinguish sarcoma-like mural nodules from anaplastic carcinomatous and sarcomatous mural nodules using accepted criteria. All mural nodules in mucinous ovarian tumors should be regarded as potentially malignant neoplasms, subject to thorough sampling and rigorous clinical correlation to exclude extraovarian spread.
This study has certain limitations which warrant discussion. First, although this is a relatively large series for this entity, the rarity of mucinous ovarian tumors with mural nodules limits cohort size. As a result, some sub-cohorts of interest (e.g., sarcoma-like mural nodules) are limited to only a few cases, and adequately powered statistical analyses are not feasible. No case in our series harbored a sarcomatous nodule only, reflecting the rarity of this subtype in the literature (see Table 3), as well as the lack of clear morphologic and immunophenotypic criteria for distinguishing a spindled anaplastic carcinomatous nodule from a sarcomatous nodule.
Because several of the cases are personal or cancer center consults, (1) the cohort may be enriched for relatively poor outcomes and chemotherapy-treated patients, (2) some cases had only select slides available for morphologic review, and (3) tissue for immunohistochemical studies was not available from all tumors. However, given the rarity of mucinous ovarian tumors with mural nodules, all published series have been largely consultation-based with limited material available for review (e.g., a median of two slides per case in one study [4]). Clinical follow-up was not available for two cases received in personal consultation. This includes the sole case of anaplastic carcinoma arising in a primary retroperitoneal mucinous neoplasm, although available data indicate that this exceptional scenario is usually, though not always, associated with poor outcome [21, 29, 33, 34].
In summary, mural nodules arising in mucinous ovarian tumors span a broad morphologic spectrum, but they are clonally derived from the associated mucinous tumor in most cases, including in mural nodules with “sarcoma-like” morphology. The molecular pathogenesis of these mural nodules is not well understood, but they appear to be genetically heterogeneous, and SWI/SNF alterations may play an role in a subset. Stage appears to be the most important prognostic factor, though definitive clinically applicable conclusions are limited due to the rarity of this entity. Collaborative multi-institutional studies with coordinated prospective evaluation are warranted to better characterize the biology and behavior of neoplastic mural nodules in mucinous ovarian tumors.
References
Prat J, Scully RE. Ovarian mucinous tumors with sarcoma-like mural nodules: a report of seven cases. Cancer. 1979;44:1332–44.
Prat J, Scully RE. Sarcomas in ovarian mucinous tumors: a report of two cases. Cancer. 1979;44:1327–31.
Prat J, Young RH, Scully RE. Ovarian mucinous tumors with foci of anaplastic carcinoma. Cancer. 1982;50:300–4.
Bague S, Rodriguez IM, Prat J. Sarcoma-like mural nodules in mucinous cystic tumors of the ovary revisited: a clinicopathologic analysis of 10 additional cases. Am J Surg Pathol. 2002;26:1467–76.
Provenza C, Young RH, Prat J. Anaplastic carcinoma in mucinous ovarian tumors: a clinicopathologic study of 34 cases emphasizing the crucial impact of stage on prognosis, their histologic spectrum, and overlap with sarcomalike mural nodules. Am J Surg Pathol. 2008;32:383–9.
Leschke H. Extraosseous giant cell tumors and explanation of their origin in young granulation tissue in cystadenoma. Virchows Arch Pathol Anat Physiol Klin Med. 1951;320:164–73.
Cheasley D, Wakefield MJ, Ryland GL, Allan PE, Alsop K, Amarasinghe KC, et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. 2019;10:3935.
Mueller JJ, Schlappe BA, Kumar R, Olvera N, Dao F, Abu-Rustum N, et al. Massively parallel sequencing analysis of mucinous ovarian carcinomas: genomic profiling and differential diagnoses. Gynecol Oncol. 2018;150:127–35.
Desouki MM, Khabele D, Crispens MA, Fadare O. Ovarian mucinous tumor with malignant mural nodules: dedifferentiation or collision? Int J Gynecol Pathol. 2015;34:19–24.
Mesbah Ardakani N, Giardina T, Amanuel B, Stewart CJ. Molecular profiling reveals a clonal relationship between ovarian mucinous tumors and corresponding mural carcinomatous nodules. Am J Surg Pathol. 2017;41:1261–6.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/college of american pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062.
Nowak JA, Yurgelun MB, Bruce JL, Rojas-Rudilla V, Hall DL, Shivdasani P, et al. Detection of mismatch repair deficiency and microsatellite instability in colorectal adenocarcinoma by targeted next-generation sequencing. J Mol Diagn. 2017;19:84–91.
Ricciuti B, Kravets S, Dahlberg SE, Umeton R, Albayrak A, Subegdjo SJ, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer. 2019;7:87.
Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol. 2000;24:1447–64.
Hamada T, Sasaguri T, Tanimoto A, Arima N, Nakano R, Miyayama H, et al. Ovarian mucinous cystadenocarcinoma with sarcoma-like mural nodules. J Surg Oncol. 1995;58:201–7.
Fujii S, Konishi I, Kobayashi F, Okamura H, Yamabe H, Mori T. Sarcoma-like mural nodules combined with a microfocus of anaplastic carcinoma in mucinous ovarian tumor. Gynecol Oncol. 1985;20:219–33.
Nakamura E, Shimizu M, Mikami Y, Kawai J, Manabe T. Ovarian mucinous cystadenocarcinoma with malignant mural nodules. Pathol Int. 1998;48:645–8.
Coatham M, Li X, Karnezis AN, Hoang LN, Tessier-Cloutier B, Meng B, et al. Concurrent ARID1A and ARID1B inactivation in endometrial and ovarian dedifferentiated carcinomas. Mod Pathol. 2016;29:1586–93.
Wang Y, Hoang L, Ji JX, Huntsman DG. SWI/SNF complex mutations in gynecologic cancers: molecular mechanisms and models. Annu Rev Pathol. 2020;15:467–92.
Chaudet K, Kem M, Lerwill M, Young RH, Mino-Kenudson M, Agaimy A, et al. SWI/SNF protein and Claudin-4 expression in anaplastic carcinomas arising in mucinous tumors of the ovary and retroperitoneum. Histopathology. 2020 https://doi.org/10.1111/his.14110.
Mhawech-Fauceglia P, Ramzan A, Walia S, Pham HQ, Yessaian A. Microfocus of anaplastic carcinoma arising in mural nodule of ovarian mucinous borderline tumor with very rapid and fatal outcome. Int J Gynecol Pathol. 2016;35:348–51.
Desouki MM, Fadare O, Kanbour A, Kanbour-Shakir A. Immunophenotype and K-RAS mutation in mucinous ovarian adenocarcinoma with mural nodule of high-grade sarcoma: case report. Int J Gynecol Pathol. 2014;33:186–90.
Mackenzie R, Kommoss S, Winterhoff BJ, Kipp BR, Garcia JJ, Voss J, et al. Targeted deep sequencing of mucinous ovarian tumors reveals multiple overlapping RAS-pathway activating mutations in borderline and cancerous neoplasms. BMC Cancer. 2015;15:415.
Kang E-Y, Cheasley D, Le Page C, Wakefield M, Antill Y, Gorringe K, et al. A refined Cut-Off for p53 immunohistochemistry improves prediction of TP53 mutation status in ovarian mucinous tumors: implications for outcome analyses (Abstract 1124). Mod Pathol. 2020;33:1077–8.
Zheng J, Geng M, Li P, Li Y, Cao Y. Ovarian mucinous cystic tumor with sarcoma-like mural nodules and multifocal anaplastic carcinoma: a case report. Int J Clin Exp Pathol. 2013;6:1688–92.
Verma RN, Bhangui GR, Rao MK. Sarcoma-like nodule in ovarian mucinous tumour (a case report). Indian J Cancer. 1993;30:38–41.
Bettinger HF. A giant cell tumour of bone in a pseudomucinous cystadenoma of the ovary. J Obstet Gynaecol Br Emp. 1953;60:230–2.
Fan YS, Thomas TM, Ip PP, Cheung AN. Osteoid-forming sarcoma-like mural nodule in a retroperitoneal mucinous cystadenocarcinoma, In: Histopathology. Vol. 49. England; 2006. p 201–4.
McFarland M, Dina R, Fisher C, McCluggage WG. Osteosarcoma as malignant mural nodule in ovarian mucinous neoplasms of intestinal type: report of 2 cases. Int J Gynecol Pathol. 2015;34:369–73.
Yang S, Wang L, Sun K. Ovarian mucinous cystic tumor associated with sarcomatous mural nodule and benign Brenner tumor: a case report and literature review. Medicine. 2019;98:e14066.
Sondergaard G, Kaspersen P. Ovarian and extraovarian mucinous tumors with solid mural nodules. Int J Gynecol Pathol. 1991;10:145–55.
Roma AA, Malpica A. Primary retroperitoneal mucinous tumors: a clinicopathologic study of 18 cases. Am J Surg Pathol. 2009;33:526–33.
Demirel D, Gun I, Kucukodaci Z, Balta AZ, Ramzy I. Primary retroperitoneal mucinous cystadenoma with a sarcoma-like mural nodule: an immunohistochemical study with histogenetic considerations and literature review. Int J Gynecol Pathol. 2013;32:15–25.
Yamazaki H, Matsuzawa A, Shoda T, Iguchi H, Kyushima N. Ovarian mucinous cystic tumor of borderline malignancy with a mural nodule of anaplastic spindle cell carcinoma: a case report. J Ovarian Res. 2013;6:86.
Okumura T, Muronosono E, Tsubuku M, Terao Y, Takeda S, Maruyama M. Anaplastic carcinoma in ovarian seromucinous cystic tumor of borderline malignancy. J Ovarian Res. 2018;11:77.
Tsujimura T, Kawano K. Rhabdomyosarcoma coexistent with ovarian mucinous cystadenocarcinoma: a case report. Int J Gynecol Pathol. 1992;11:58–62.
Hillesheim PB, Farghaly H. Anaplastic spindle cell carcinoma, arising in a background of an ovarian mucinous cystic tumor: a case report with clinical follow up, review of the literature. Int J Clin Exp Pathol. 2010;3:808–11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Chapel, D.B., Lee, E.K., Da Silva, A.F.L. et al. Mural nodules in mucinous ovarian tumors represent a morphologic spectrum of clonal neoplasms: a morphologic, immunohistochemical, and molecular analysis of 13 cases. Mod Pathol 34, 613–626 (2021). https://doi.org/10.1038/s41379-020-0642-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0642-9
This article is cited by
-
An integrated machine learning-based model for joint diagnosis of ovarian cancer with multiple test indicators
Journal of Ovarian Research (2024)