Abstract
Acral lentiginous melanoma (ALM) is a rare tumor that occurs on non-sun exposed skin areas of the hands and feet. Reports suggest that ALM exhibits poor prognosis, although mechanisms driving this remain poorly understood. Alterations in TERT and the Wnt/β-catenin (Wnt) pathway have been suggested to correlate with prognosis of ALM. Thus, immunohistochemical expression of β-catenin and LEF1 along with TERT amplification by FISH was investigated in 34 primary ALMs, 20 metastatic ALMs, 10 primary non-ALMs, and 15 acral nevi. Foot/toe was the most common primary tumor location (85%) for ALM. TERT amplification was detected in 6 of 28 (21.4%) primary ALM, 2 of 8 (25%) primary non-ALM, and 8 of 18 (44.4%) metastatic ALM, the latter showing significantly higher frequency compared with primary melanomas (P = 0.043). Most metastatic ALMs positive for TERT amplification lacked BRAF V600E (87.5%). Cytoplasmic and nonnuclear expression of β-catenin was variably detected in all cases. Metastatic ALM revealed lower expression of β-catenin compared with primary ALM (P = 0.017). No differences in LEF1 expression were detected among the groups; however, acral nevi showed decreased labeling with dermal descent, in contrast to melanoma. No molecular-genetic alteration correlated with prognosis. TERT amplification by FISH is a frequent finding in primary ALM and appears to increase in metastatic tumors, suggesting a role in tumor progression to metastasis. Although TERT amplification has been reported to be infrequent in primary non-ALM, it showed comparable frequency with ALM in our series. Our immunohistochemical findings are not fully supportive of activation of either canonical or noncanonical Wnt cascades in ALM. TERT amplification by FISH and LEF1 immunohistochemistry may help in the differential diagnosis between primary ALM and acral nevus. TERT amplification appears to be a promising target for therapy in patients with metastatic ALM.
Similar content being viewed by others
Introduction
Acral lentiginous melanoma (ALM) is an uncommon subtype of cutaneous melanoma, more frequently seen in non-Caucasians [1, 2]. ALM accounts for ~4% of all melanomas in white populations. The disease occurs on acral skin like palms and soles. ALM is known to have a higher frequency of chromosomal aberrations and is regarded to have a worse prognosis than the more common non-acral cutaneous melanoma [3,4,5]. ALM is also thought to be less responsive to immune checkpoint inhibitors because of the poor immune response to the tumor [6, 7].
Approximately 50% of all cutaneous melanomas harbor activating BRAF gene mutations, making these the most frequent mutations in melanoma. More than 90% of BRAF mutations in melanoma occur at codon 600 (BRAF V600E). In contrast, ALMs show low frequency of about 15–20% BRAF V600E mutations. Mutations in genes such as KIT, PDGFRA, and NRAS appear to occur more frequently in ALM [8,9,10].
TERT is a gene encoding for the catalytic subunit of telomerase reverse transcriptase. Upregulation of TERT activity enhances cellular proliferation and plays an important role in oncogenesis. TERT promoter mutations occur early in melanomagenesis and UV exposure is probably the key mechanism for the generation of these aberrations. TERT promoter mutations are associated with poor prognosis in primary and metastatic melanoma; however, they are reported to be uncommon in ALM. In contrast, TERT has been reported to be involved in frequent amplifications of small genomic regions in primary ALM [11,12,13]. TERT gene amplification is another mechanism for TERT gene activation that promotes cell survival and proliferation [14,15,16,17] and has been found to be associated with poor prognosis in breast and urothelial carcinomas [18] and with an aggressive behavior in lung adenocarcinoma, glioblastoma, meningioma, and neuroblastoma [19, 20]. Compared with TERT promoter mutations, TERT amplifications are found in a small number of malignancies [18]. TERT gene amplification has been detected in 21% of cases of primary ALM and reported to be associated with poor outcome in a single study [21], however, its incidence in primary non-ALM has not been extensively explored [22].
Besides maintaining telomere length, telomerase appear to have other roles. High telomerase expression has been proposed to influence both phenotype and metabolism of cancer cells by interacting with Wnt signaling cascades [23]. Activation of the Wnt/β-catenin signaling pathway is a prognostic biomarker and a therapy target in several tumor types [24]. β-catenin and LEF1 are central mediators of the Wnt/β-catenin signaling pathway. In the absence of a Wnt signal, a degradation complex phosphorylates β-catenin, targeting it for elimination. In the presence of a Wnt signal, β-catenin is stabilized in the cytoplasm. The cytoplasmic accumulation of β-catenin leads to its translocation to the nucleus, where it binds to LEF1 and activates transcription of a number of target genes [25]. LEF1 and β-catenin protein expression are promising biomarkers that have been reported to be associated with poor prognosis in a subset of ALMs [26]; however, besides this single report, little is known of the significance of β-catenin and LEF1 in the progression and prognosis of ALM.
The aim of this study was to determine the significance of biomarkers suggested to play a role in ALM, such as TERT gene amplification and protein expression of β-catenin and LEF1, in progression of primary ALM to metastasis and its prognosis.
Materials and methods
The study was approved by the Institutional Ethics Review Board. Seventy-nine formalin-fixed, paraffin embedded samples of acral and non-acral melanocytic lesions were collected from 2005 to 2018 from the pathology department at our hospital. The cases included specimens collected at our institution and those sent to our institution in consultation. Pathology reports were evaluated for histopathologic parameters. Clinical data and results of genetic testing of common mutations were retrieved from review of the clinical files. The cases were divided into different categories with four groups including primary ALM (n = 34), primary non-ALM (n = 10), metastatic ALM (n = 20), and acral melanocytic nevi (n = 15). Cases of primary and metastatic ALM corresponded to different patients.
Immunohistochemistry for β-catenin, LEF1, and BRAF V600E and fluorescence in situ hybridization (FISH) for TERT gene amplification were performed in all the cases. Immunohistochemical analysis using an anti-β-catenin monoclonal mouse antihuman antibody (dilution 1:1500, clone 14, Dako Cytomation, Carpinteria, CA) and an anti-LEF1 monoclonal rabbit antihuman antibody (dilution 1:100, clone EPR2029y, Dako Cytomation) was performed on paraffin sections according to the BOND MAX protocol (Vision Biosystems, Norwell, MA) guidelines. For BRAFV600E, (Ventana anti-BRAF V600E (VE1) mouse monoclonal antibody, Tucson, Arizona) was used as primary antibody. For β-catenin and LEF1, intensity (0, 1+, 2+, and 3+), number of positive cells (%) and cytoplasmic versus nuclear expression were recorded and H-score was calculated. Briefly, the H-score was determined according to the formula: [H-score = (%1+ × 1) + (%2+ × 2) + (%3+ × 3)]. BRAF V600E immunohistochemical staining was interpreted as positive or negative. The slides were scored as positive when most of the tumor cells showed moderate to strong cytoplasmic staining and they were considered negative when there was no staining or weak staining of single interspersed cells. Appropriate positive and negative controls were used for each antibody.
For FISH analysis, a commercial probe (about 390 kB) covering the TERT gene at 5p15 and a control probe (about 650 kB) covering 5q31 (including CDC25C and EGR1 genes) were used (Leica Biosystems-Kreatech, Amsterdam, Netherlands; Fig. 1). Gene amplifications were considered positive when the ratio between TERT gene copy number and control was greater than 1.11. This cutoff criteria was established by the Cytogenetics Training Laboratory at the School of Health Professions, MD Anderson Cancer Center through probe validation and statistical analysis on a set of normal tissue samples. Some cases were not interpretable and therefore were excluded from analysis. All samples were de-paraffinized and pretreated following manufacturer’s specifications before probe application (Agilent-Dako, Santa Clara, CA).
Statistical analysis was done using SPSS software version 24 (IBM Corp., Armonk, NY). Chi square and t tests with descriptive statistics were used for statistical analysis, and the results were considered statistically significant at P < 0.05.
Results
Clinicopathological characteristics of patients
The main clinical characteristics of the ALM and non-ALM patients are listed in Table 1. Median age and sex were comparable among ALM, non-ALM, and metastatic ALM groups. In the ALM melanomas (primary and metastatic), the tumor occurred on the foot/toe in 44 of 54 patients (81.5%) and on the hands/fingers in 5 of 54 patients (9.3%). There was a trend for worse average survival in ALM as compared with non-ALM, however it did not reach statistical significance (P = 0.17).
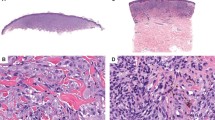
The main histopathological features of the groups are listed in Table 2 and shown in Fig. 2. The median Breslow thickness for primary ALM (Fig. 2a) was 2.75 mm (range, 0.8–16.0 mm) and for non-ALM was 1.8 mm (range, 0.95–5 mm). ALM and non-ALM were similarly distributed among AJCC 8th Edition T categories based on Breslow thickness [27]. Ulceration was present in 17 of 34 ALM melanomas (50%) and 2 of 10 non-ALM (20%). Median mitotic count was 2 mitoses/mm2 (range, <1–21 mitoses/mm2) for ALM and was 2 mitoses/mm2 (range, <1–7 mitoses/mm2) for non-ALM. In our study, for both ALM and non-ALM melanoma groups, there was a trend for thicker melanomas (high Breslow thickness) and more than 1 mitosis/mm2 to be associated with worse overall survival (P = 0.19, and P = 0.08, respectively), however there was no survival difference for ulceration (P = 0.66).
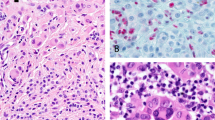
a Primary acral lentiginous melanoma. The tumor shows invasive epithelioid melanoma cells with marked cytologic atypia (H&E, ×20). b Acral melanocytic nevus, compound. Notice the very focal junctional component and maturation of the dermal cells with descent. (H&E, ×20). c LEF1 immunohistochemical expression in primary ALM. Strongly nuclear expression is seen throughout the tumor (immunohistochemistry ×200). d Acral compound melanocytic nevus showing decreased nuclear positivity of LEF1 with descent (“maturation pattern”) of LEF1 (immunohistochemistry, ×40). e Cytoplasmic/membranous expression of β-catenin in primary ALM (immunohistochemistry, ×200). f Decreased cytoplasmic/membranous expression of β-catenin in metastatic ALM (immunohistochemistry, ×40). g About 20% of our cases of primary ALM demonstrated BRAF V600E expression (immunohistochemistry, ×200). h Acral melanocytic nevus showing positive for BRAF V600E expression. About 67% of our cases were positive for BRAF V600E (immunohistochemistry, ×40). i Acral compound melanocytic nevus showing two signals per nuclei for TERT, indicating the absence of amplification (red: TERT at 5p15.3; green: 5q31). j TERT amplification by FISH in a case of metastatic acral lentiginous melanoma (red: TERT at 5p15.3; green: 5q31).
Our 15 patients with acral nevi included 4 men and 11 women (male-to-female ratio, 0.4:1); the median age was 48 years (range, 26–71 years). Most of the lesions (11 of 15; 73%) occurred on the foot/toe and histologically seven of the lesions were compound (Fig. 2b).
Immunohistochemistry results
LEF1 demonstrated nuclear expression in all our positive cases. No cytoplasmic LEF1 labeling was found in melanoma, as opposite to what has been reported in the literature [26] (Fig. 2c). All of our compound acral nevi (7 of 7; 100%) showed decreased nuclear positivity of LEF1 with the descent (i.e., LEF1 appeared to show a “maturation pattern”, Fig. 2d). In contrast, 42 of 44 melanomas (95%) showed patchy LEF1 positivity in the dermal component of the tumors (Fig. 2c). No significant correlation between LEF1 and type of melanoma (ALM versus non-ALM), primary versus metastatic tumors, clinicopathologic characteristics, or prognosis was found.
β-catenin expression was cytoplasmic in melanoma and nevi, with no cases showing nuclear β-catenin labeling. The H-score of β-catenin in metastatic ALM melanomas was significantly lower than that seen in primary ALM (P = 0.017, Table 3 and Fig. 2e, f). The same difference was present when metastatic ALM was compared with primary ALM and non-ALM as one group (P = 0.014). However, there was no significant correlation between β-catenin expression and prognosis.
Seven of 34 (20.6%) ALM and 10 of 15 (66.7%) acral nevi were positive for BRAF V600E by immunohistochemistry. Acral nevi showed a much higher frequency of BRAF V600E mutation as compared with ALMs (P = 0.05, Fig. 2g, h).
FISH results
TERT amplification was detected by FISH in 6 of 28 (21.4%) of primary ALMs, 2 of 8 (25%) of primary non-ALMs, and 8 of 18 (44.4%) of metastatic ALMs. When comparing primary with metastatic ALM, it was noted that the metastatic tumors showed a significantly higher frequency of TERT amplification (P = 0.043). This was also true when comparing primary melanoma (ALM plus non-ALM) with metastatic ALM (P = 0.022).
All of the acral melanocytic nevi tested showed lack of TERT amplification (12 of 12; 100%). A statistically significant difference in TERT amplification by FISH was detected between acral melanocytic nevi and melanoma.
Comparison of clinicopathological characteristics of patients with melanomas harboring TERT amplification revealed no differences, including outcome, among ALM and non-ALMs.
Gene mutation analysis results
Gene mutation analysis for the melanomas and acral nevi are summarized in Table 4. In our study, 7 of 34 (20%) ALMs, 3 of 10 (30%) non-ALMs, 2 of 20 (10%) metastatic ALM, and 10 of 15 (66.7%) acral nevi harbored BRAF V600E, detected by gene sequencing or immunohistochemistry. The percentage of cases positive for BRAF V600E detected in acral nevi and ALM was significantly different (P = 0.05).
Among ALMs, besides clinical testing for common mutations (BRAF, KIT, and NRAS, Table 4), next generation sequencing (NGS) analysis of 23 tumors (12 metastases and 11 primaries) revealed only one case (4.3%, primary ALM) harboring mutations in TERT and NF1, one with MDM2, PIK3CA, and BIRC2 mutations, one with SMARCB1 and TP53 mutations, one with VHL and TP53 mutations, one with NOTCH2 and CDK6 mutations, one with a JAK3 mutation, and 17 without evidence of any tested mutation.
No correlation between gene mutations and type of melanoma, clinicopathological features, or prognosis was found.
Discussion
Although ALM occurs less frequently that cutaneous melanoma, ALM comprises a relatively high proportion of cutaneous melanoma in non-Caucasians, in whom cutaneous melanomas occur less frequently [1]. The most common primary anatomical site for ALM in our study was the foot/toe. ALM is commonly regarded as a subtype harboring worse prognosis than the other subtypes of melanoma [4]. In our series, we found a trend for these patients to show worse overall survival although this difference did not reach statistical significance. Our small control group sample size (n = 10) may account for this finding.
The immunohistochemical expression of LEF1 and β-catenin, markers of the Wnt/β-catenin signaling pathway, were analyzed in relation to clinicopathological characteristics, tumor type (primary ALM versus non-acral and primary versus metastatic), and prognosis. We did not detect any differences in immunohistochemical nuclear expression of LEF1 among primary and metastatic ALM, between primary ALM versus non-ALM, or between melanomas and acral nevi. In contrast, β-catenin cytoplasmic expression in metastatic ALM was significantly lower than that of primary melanomas. Detection of cytoplasmic β-catenin in acral melanocytic nevi was similar to that of primary melanoma. In our study, we observed nuclear expression of LEF1 in all of the melanocytic lesions studied. In contrast, LEF1 expression in ALM in a previous study [26] was reported as consistently cytoplasmic. Nuclear β-catenin labeling was not detected in any of our cases, including acral melanocytic nevi, suggesting that LEF1 may act independently of nuclear localization of β-catenin in acral melanocytic lesions. Our overall immunohistochemical findings are not supportive of activation of the canonical Wnt pathway in ALM, in contrast to what is described in other types of melanoma, where the translocation of β-catenin to the nucleus and its subsequent binding to LEF1 leads to activation of other target genes such as c-MYC and CCND1 [28]. While nuclear localization of LEF1 and decreased cytoplasmic expression of β-catenin in metastases may suggest activation of a noncanonical Wnt pathway in ALM, our immunohistochemical data does not fully support this contention either. It is also possible that immunohistochemistry may not be a reliable indicator of Wnt pathway in ALM.
Our analysis revealed that all of our compound acral nevi showed nuclear expression of LEF1 in junctional and superficial dermal melanocytes with progressive decrease of expression with descent into the dermis. This finding may be in relation to low proliferation and activation of senescence pathways present in these lesions. In contrast to acral nevi, most of our melanomas showed patchy, random positivity in the invasive dermal cells, including in deeper aspect of the dermis. On practical grounds, evaluation of the immunohistochemical pattern of expression of LEF1 may help in the evaluation of acral melanocytic lesions, although variations in this pattern of expression in both benign and malignant acral melanocytic lesions are expected.
LEF1 and β-catenin immunohistochemical expression did not correlate with clinicopathological parameters or prognosis in our melanoma cases, contrary to a prior report [26]. This lack of correlation persisted when BRAF V600E status was included in the analysis. This finding may support lack of clear association between canonical Wnt pathway and ALM. Analysis of larger cohorts of ALM and non-ALM cases may help elucidate the role of these biomarkers in prognosis.
Overall 67% of acral nevi were BRAF V600E positive, supporting that BRAF mutations are major events involved in the genesis of melanocytic nevus, even in sun-protected areas [9]. Among ALM, only 21% were BRAF V600E positive. The BRAF V600E status between ALM and acral nevus showed a nearly significant difference (P = 0.05). This finding may also support the hypothesis that ALMs are less likely to arise from acral melanocytic nevi as compared with other cutaneous melanomas, and that most ALM arise de novo [29].
We found that primary ALMs harbor TERT gene amplifications in 21.4% of cases. This is in agreement with the only two previous reports exploring this phenomenon in primary ALM, where the range of TERT amplification was between 20.1% and 29.4% [18, 21, 30]. In a cohort of tumors from The Cancer Genome Atlas database it was found that 6% of melanomas (without specifying subtype) showed TERT amplification by NGS methodologies [31] while less than 5% of desmoplastic melanomas revealed focal amplifications of the gene, in another series [32]. Utilizing FISH, four out of ten cases (40%) of melanoma (designated as conventional, Spitzoid, and melanomas arising in giant congenital nevi) were found to show TERT amplification [33]. Our finding of 25% of primary non-ALMs showing TERT amplification adds to the evidence that type of melanoma and methodology used (likely related to different cutoffs for copy number alterations) may account for variations in frequency of TERT amplification in primary melanoma.
None of the acral nevi tested were found to have TERT gene amplification. In cases of ambiguous lesions where melanoma and nevus are in the differential diagnosis, a combination of TERT amplification results and pattern of expression of LEF1, as indicated above, may be of utility. TERT gene amplification has been already reported as a promising tool for differentiating malignant from benign acral melanocytic tumors [14].
In our study, metastatic ALMs showed a higher frequency of TERT amplification as compared with primary ALM (P = 0.043), although a significant association between TERT amplification and overall survival was not detected. While TERT amplification has been previously reported in primary ALM and thought to be overall rare in other types of primary melanoma, higher frequency of TERT amplification in metastatic ALM in relation to primary tumors have not been previously reported, to our knowledge. Our findings may suggest that TERT amplification is a common event implicated in progression to metastasis in ALM. This phenomenon may not be exclusive of ALM since metastatic non-ALM cases were not included in this study. Adding more evidence to this hypothesis, TERT amplifications have been detected in circulating melanoma cells in patients with advanced stage melanoma [34]. This phenomenon seems to differ from what is currently known about TERT promoter mutations in melanoma. First, similar proportions of TERT promoter mutations have been found in primary and metastatic cutaneous melanoma, along with lack of correlation with adverse tumor characteristics [35]. Second, in a series of primary and corresponding metastatic melanomas [36], it was reported that a greater proportion of metastases were TERT wild type (71% vs. 29% showing TERT promoter mutation) and of these, 62% lost their TERT promoter mutation in relation to the primary tumor. This finding was hypothesized to indicate that in at least a subset of melanomas, TERT promoter mutations may not be essential to disease progression. TERT amplification, on the other hand, appears to have a role in the progression from primary to metastatic ALM. This is further supported by the fact that only one case (4.3%, a primary tumor) out of 23 primary and metastatic ALMs tested by NGS was found to harbor TERT promoter mutations in our series.
In our study, 14/16 (87.5%) TERT amplified cases were BRAF wild type. This finding is in keeping with prior reports showing that tumors with TERT abnormalities are BRAF wild type [37]. In clinical grounds, this observation is important since these patients are not eligible for targeted therapy with BRAF inhibitors. As TERT is a key player in melanoma, it can therefore be an alternate target in these patients who may lack other treatment options. Most of the TERT inhibitors evaluated so far have prolonged lag period of efficacy due to their reliance on targeting the telomerase enzyme, potentially allowing cancer cells to adapt and also increasing their toxicity. However, in addition to its canonical role in maintaining telomere length, TERT also regulates extratelomeric processes like apoptosis, chromatin state, cell proliferation, and DNA damage responses. Hence, other novel TERT-based therapeutic strategies may also be promising and useful for rapid and sustained cancer treatment response [38].
In summary, we report that TERT amplification is detected in primary ALM and the frequency of this genetic event increases in metastatic tumors, indicated a possible role in tumor progression to metastasis. TERT amplifications were also found to be frequent (25%) in primary non-ALM, in contrast to previous reports. No support for activation of canonical Wnt pathway in ALM was observed, although decreased cytoplasmic expression of β-catenin was seen in metastatic ALM. Along with TERT amplification detection by FISH, LEF1 immunohistochemistry may help in the differential diagnosis between ALM and nevus. For patients with metastatic ALM, whose tumors are frequently BRAF wild type, TERT amplification, and noncanonical Wnt pathways appear to be promising targets for therapy.
References
Bradford PT, Goldstein AM, McMaster ML, Tucker MA. Acral lentiginous melanoma: incidence and survival patterns in the United States, 1986–2005. Arch Dermatol. 2009;145:427–34.
Pollack LA, Li J, Berkowitz Z, Berkowitz Z, Weir HK, Wu XC, et al. Melanoma survival in the United States, 1992 to 2005. J Am Acad Dermatol. 2011;65:S78–86.
Goydos JS, Shoen SL. Acral lentiginous melanoma. Cancer Treat Res. 2016;167:321–9.
Bello DM, Chou JF, Panageas KS, Brady MS, Coit DG, Carvajal RD, et al. Prognosis of acral melanoma: a series of 281 patients. Ann Surg Oncol. 2013;20:3618–25.
Pradhan D, Jour G, Milton D,Vasudevaraja V, Tetzlaff MT, Nagarajan P, et al. Aberrant DNA methylation predicts melanoma-specific survival in patients with acral melanoma. Cancers (Basel). 2019;11.
Kato J, Hida T, Someya M, Sato S, Sawada M, Horimoto K, et al. Efficacy of combined radiotherapy and anti-programmed death 1 therapy in acral and mucosal melanoma. J Dermatol. 2019;46:328–33.
Nakamura Y, Fujisawa Y. Diagnosis and management of acral lentiginous melanoma. Curr Treat Options Oncol. 2018;19:42.
Vazquez Vde L, Vicente AL, Carloni A, Berardinelli G, Soares P, Scapulatempo C, et al. Molecular profiling, including TERT promoter mutations, of acral lentiginous melanomas. Melanoma Res. 2016;26:93–9.
Moon KR, Choi YD, Kim JM, Jin S, Shin MH, Shim HJ, et al. Genetic alterations in primary acral melanoma and acral melanocytic nevus in Korea: common mutated genes show distinct cytomorphological features. J Investig Dermatol. 2018;138:933–45.
Torres-Cabala CA, Wang WL, Trent J, Yang D, Chen S, Galbincea J, et al. Correlation between KIT expression and KIT mutation in melanoma: a study of 173 cases with emphasis on the acral-lentiginous/mucosal type. Mod Pathol. 2009;22:1446–56.
Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–47.
Liau JY, Tsai JH, Jeng YM, Chu CY, Kuo KT, Liang CW. TERT promoter mutation is uncommon in acral lentiginous melanoma. J Cutan Pathol. 2014;41:504–8.
Seynnaeve B, Lee S, Borah S, Park Y, Pappo A, Kirkwood JM, et al. Genetic and epigenetic alterations of TERT are associated with inferior outcome in adolescent and young adult patients with melanoma. Sci Rep. 2017;7:45704.
Diaz A, Puig-Butille JA, Valera A, Muñoz C, Costa D, Garcia-Herrera A, et al. TERT and AURKA gene copy number gains enhance the detection of acral lentiginous melanomas by fluorescence in situ hybridization. J Mol Diagn. 2014;16:198–206.
Griewank KG, Murali R, Puig-Butille JA, Schilling B, Livingstone E, Potrony M, et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst. 2014;106.
Thomas NE, Edmiston SN, Tsai YS, Parker JS, Googe PB, Busam KJ, et al. Utility of TERT promoter mutations for cutaneous primary melanoma diagnosis. Am J Dermatopathol. 2019;41:264–72.
Xie H, Liu T, Wang N, Björnhagen V, Höög A, Larsson C, et al. TERT promoter mutations and gene amplification: promoting TERT expression in Merkel cell carcinoma. Oncotarget. 2014;5:10048–57.
Gaspar TB, Sa A, Lopes JM, Sobrinho-Simões M, Soares P, Vinagre J. Telomere maintenance mechanisms in cancer. Genes. 2018;9.
Zhu CQ, Cutz JC, Liu N, Lau D, Shepherd FA, Squire JA, et al. Amplification of telomerase (hTERT) gene is a poor prognostic marker in non-small-cell lung cancer. Br J Cancer. 2006;94:1452–9.
Valentijn LJ, Koster J, Zwijnenburg DA, Hasselt NE, van Sluis P, Volckmann R, et al. TERT rearrangements are frequent in neuroblastoma and identify aggressive tumors. Nat Genet. 2015;47:1411–4.
Diaz A, Puig-Butille JA, Munoz C, Costa D, Díez A, Garcia-Herrera A, et al. TERT gene amplification is associated with poor outcome in acral lentiginous melanoma. J Am Acad Dermatol. 2014;71:839–41.
Motaparthi K, Kim J, Andea AA, Missall TA, Novoa RA, Vidal CI, et al. TERT and TERT promoter in melanocytic neoplasms: current concepts in pathogenesis, diagnosis, and prognosis. J Cutan Pathol. 2020. https://doi.org/10.1111/cup.13691. [Epub ahead of print]
Ofner R, Ritter C, Heidenreich B, Kumar R, Ugurel S, Schrama D, et al. Distribution of TERT promoter mutations in primary and metastatic melanomas in Austrian patients. J Cancer Res Clin Oncol. 2017;143:613–7.
Santiago L, Daniels G, Wang D, Deng FM, Lee P. Wnt signaling pathway protein LEF1 in cancer, as a biomarker for prognosis and a target for treatment. Am J Cancer Res. 2017;7:1389–406.
Centelles JJ. General aspects of colorectal cancer. ISRN Oncol. 2012;2012:139268.
Xu S, Yang Z, Zhang J, Jiang Y, Chen Y, Li H, et al. Increased levels of beta-catenin, LEF-1, and HPA-1 correlate with poor prognosis for acral melanoma with negative BRAF and NRAS mutation in BRAF exons 11 and 15 and NRAS exons 1 and 2. DNA Cell Biol. 2015;34:69–77.
Kanaki T, Stang A, Gutzmer R, Zimmer L, Chorti E, Sucker A, et al. Impact of American Joint Committee on Cancer 8th edition classification on staging and survival of patients with melanoma. Eur J Cancer. 2019;119:18–29.
Dantonio PM, Klein MO, Freire M, Araujo CN, Chiacetti AC, Correa RG. Exploring major signaling cascades in melanomagenesis: a rationale route for targetted skin cancer therapy. Biosci Rep. 2018;38.
Sheen YS, Liao YH, Lin MH, Chen JS, Liau JY, Liang CW, et al. Clinicopathological features and prognosis of patients with de novo versus nevus-associated melanoma in Taiwan. PLoS One. 2017;12:e0177126.
Puig-Butille JA, Badenas C, Ogbah Z, et al. Genetic alterations in RAS-regulated pathway in acral lentiginous melanoma. Exp Dermatol. 2013;22:148–50.
Barthel FP, Wei W, Tang M, Carrera C, Aguilera P, Malvehy J, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49:349–57.
Shain AH, Garrido M, Botton T, Talevich E, Yeh I, Sanborn JZ, et al. Exome sequencing of desmoplastic melanoma identifies recurrent NFKBIE promoter mutations and diverse activating mutations in the MAPK pathway. Nat Genet. 2015;47:1194–9.
Lee S, Opresko P, Pappo A, Kirkwood JM, Bahrami A. Association of TERT promoter mutations with telomerase expression in melanoma. Pigment Cell Melanoma Res. 2016;29:391–3.
Ruiz C, Li J, Luttgen MS, Kolatkar A, Kendall JT, Flores E, et al. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys Biol. 2015;12:016008.
Hugdahl E, Kalvenes MB, Mannelqvist M, Ladstein RG, Akslen LA. Prognostic impact and concordance of TERT promoter mutation and protein expression in matched primary and metastatic cutaneous melanoma. Br J Cancer. 2018;118:98–105.
Yang S, Leone DA, Biswas A, Deng A, Jukic D, Singh R, et al. Concordance of somatic mutation profiles (BRAF,NRAS, and TERT) and tumoral PD-L1 in matched primary cutaneous and metastatic melanoma samples. Hum Pathol. 2018;82:206–14.
Liang WS, Hendricks W, Kiefer J, Schmidt J, Sekar S, Carpten J, et al. Integrated genomic analyses reveal frequent TERT aberrations in acral melanoma. Genome Res. 2017;27:524–32.
Reyes-Uribe P, Adrianzen-Ruesta MP, Deng Z, Echevarria-Vargas I, Mender I, Saheb S, et al. Exploiting TERT dependency as a therapeutic strategy for NRAS-mutant melanoma. Oncogene. 2018;37:4058–72.
Acknowledgements
This project was supported by the N. Allen and Barbara B. Kannapell Fund for Melanoma Oncology Research. Editorial support was provided by Bryan Tutt in Scientific publication Services, Research Medical Library, The University of Texas MD Anderson Cancer Center, Houston, TX.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ramani, N.S., Aung, P.P., Gu, J. et al. TERT amplification but not activation of canonical Wnt/β-catenin pathway is involved in acral lentiginous melanoma progression to metastasis. Mod Pathol 33, 2067–2074 (2020). https://doi.org/10.1038/s41379-020-0565-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0565-5
This article is cited by
-
Extensive review on breast cancer its etiology, progression, prognostic markers, and treatment
Medical Oncology (2023)
-
Prognostic significance of acral lentiginous histologic type in T1 melanoma
Modern Pathology (2021)