Abstract
Medullary thyroid carcinoma (MTC) is a rare nonfollicular cell-derived tumor. A robust grading system may help better stratify patients at risk for recurrence and death from disease. In total, 144 MTC between 1988 and 2018 were subjected to a detailed histopathologic evaluation. Clinical and pathologic data were correlated with disease specific survival (DSS), local recurrence free survival (LRFS) and distant metastasis free survival (DMFS). Median age was 53 years (range: 3–88). Median tumor size was 1.8 cm (range: 0.2–11). Lymph node metastases were present in 84 (58%) cases while distant metastases at presentation were found in 9 (6%) patients. Seven (5%) had ≥5 mitoses/10 HPFs. Tumor necrosis was present in 30 cases (20%) while lymphovascular invasion occurred in 41 (28%) of tumors. Extra-thyroidal extension was found in 44 (31%) and positive margins were seen in 19 (14%). There was a strong correlation between increasing tumor size and tumor necrosis (p < 0.001). Median follow up was 39 months. In univariate analysis, male gender, higher American Joint Committee on Cancer (AJCC) stage group, larger tumor size, tumor necrosis, high mitotic index (≥5/10 HPF), nodal status, size of largest nodal metastasis, and elevated postoperative serum calcitonin predicted worse DSS, LRFS, and DMFS (p < 0.05). Extra-thyroidal extension correlated with DSS and DMFS while positive margins and distant metastasis at presentation imparted worse DSS (p < 0.05). In multivariate analysis, tumor necrosis and mitotic activity (5 mitosis/10 HPFs as the cutoff) were the only independent predictors for DSS (p = 0.008 and 0.026, respectively). Tumor necrosis was the sole independent prognostic factor for LRFS and DMFS (p = 0.001 and 0.003, respectively). The presence of tumor necrosis and high mitotic rate are powerful independent prognostic factors in MTC and outperform serum calcitonin and stage. We propose a grading system based on tumor necrosis and mitotic activity to better stratify MTC patients for counseling, post-resection surveillance, and therapy.
Similar content being viewed by others
Introduction
Medullary thyroid carcinoma (MTC) is rare accounting for 1–2% of all thyroid malignancies [1]. These tumors originate from the neural crest derived parafollicular C cells of the thyroid gland that secrete calcitonin [2, 3]. As in many thyroid carcinomas, the clinical course of patients with MTC is variable. Several prognostic factors were shown to confer worse outcome such as age, sex, TNM stage, sporadic versus hereditary disease, distant metastasis, nodal metastatic burden, serum calcitonin, carcinoembryonic antigen (CEA) blood levels, somatic RET mutation status, response to initial therapy risk stratification and extent of thyroidectomy [4,5,6,7,8]. However, the predictive value of some of these parameters such as age is controversial [9]. Novel and robust prognostic markers of outcome are needed to help guide surgical decision making, systemic therapy, and post-resection surveillance strategies.
Despite the initial report by Jacquet of a thyroid tumor with amyloid more than 100 years ago and since the definite histologic description of MTC by Hazard et al. in 1959, there has never been a histologic grading system for this entity [10, 11]. This is surprising since MTC is a neuroendocrine neoplasm and neuroendocrine tumors (NET) in other sites such as lung, pancreas, and gastrointestinal tract are usually amenable to grading mainly based on mitotic count, tumor necrosis, and/or Ki-67 proliferation index [12, 13]. In order to develop a grading scheme for MTC, we performed a detailed histopathologic review of 144 MTCs and correlated the histologic parameters with outcome and other clinical features. We sought to determine if tumor grade, as measured by mitotic count and/or tumor necrosis would better predict disease-specific outcomes.
Material and methods
Study population
The cancer registry at Memorial Sloan Kettering Cancer Center (MSKCC) was searched for all patients with a pathologic diagnosis of MTC, who underwent thyroidectomy between 1986 and 2017. Slides from the primary tumor were available for review by the study pathologists (RAG, BA) on 144 patients. Of note, all the slides were examined by a senior head and neck pathologist with a special interest in thyroid neoplasia (R.A.G.). The pathologists were blinded to the patients’ outcome. The study was approved by the Institutional Review Board of MSKCC.
Histopathologic examination
The diagnosis of MTC was based on the morphologic appearance and/or immunohistochemical profile of the tumor. The MTC was subtyped as classical or other variants on the basis of the most recent WHO classification of endocrine neoplasms [13]. The largest dimension of the tumor was based upon a reconciliation of the gross and microscopic findings. The largest size of the largest tumor nodule was used in the analysis. Mitotic rate and tumor necrosis were assessed in the initial thyroidectomy resection and attached lymph nodes. The mitotic rate of the carcinoma was determined by counting 10 contiguous high-power fields (400×) using an Olympus microscope (U-DO model BX-40; Olympus America Inc., Melville, NY). Using that microscope type, 10 high-power fields correspond to 2.4 mm2. Mitotic counts were performed in a focused fashion, examining areas that appeared to show greater proliferative activity (so called hot spots). Spontaneous tumor necrosis was classified as absent or present. Tumor necrosis was defined by a “comedo-like” appearance composed of degenerating cytoplasm and punctuate, karyorrhectic nuclear debris (Figs. 1, 2). The presence of fibroblastic stromal reaction, hemorrhage or an identifiable needle track in the necrotic area was attributable to reactive changes induced by prior fine needle aspiration and was therefore not labeled as spontaneous tumor necrosis. Nuclear pleomorphism of the carcinoma cells was characterized as absent/mild, moderate or marked. The presence or absence of amyloid in the tumor was recorded. Intratumoral fibrosis was classified as absent/mild, moderate or prominent. In regard to the status of the tumor capsule, the carcinoma was categorized as completely encapsulated/well circumscribed, partially encapsulated or totally lacking a capsule. Capsular invasion was defined as complete penetration of the capsule by tumor. Only lymphovascular invasion (LVI) of thyroid or extra-thyroid soft tissue vessels was included in the analysis. LVI was diagnosed in accordance with the criteria outlined by the Armed Forces Institute of Pathology fascicle and the WHO classification of endocrine tumors [13, 14]. Only when the invasive focus was present in the vascular lumen covered by endothelial cells, or when it was attached to the vessel wall protruding into the lumen of the vessel in a polypoid manner or associated with thrombus formation, it was considered as LVI. For encapsulated tumors, it was defined as invasion of a vessel within or outside the tumor capsule. If the tumor was not encapsulated, any LVI inside or outside the tumor was considered as LVI. The foci of capsular and LVI were counted and subdivided into two categories: focal (<4 invasive foci) and extensive (≥4 foci). A tumor was deemed infiltrative if the carcinoma cells invaded in between non-neoplastic thyroid follicles. Extra-thyroidal extension was defined as invasion of peri-thyroid adipose tissue, skeletal muscle or adjacent organs. A surgical margin was considered as positive if tumor was present at the inked resection edge. The number of separate foci of MTC was documented. The number of lymph nodes examined and those with metastasis was recorded along with the size of the largest node positive for tumor, the size of the largest metastatic focus and the presence of extra-nodal extension. The presence of C-cell hyperplasia was documented when feasible.
Patient was M0 at presentation with postoperative serum calcitonin and CEA of 898 pg/ml and 2.4 ng/ml, respectively. The patient died of disease 2 years and 4 months after initial surgery. a Medium power view of an H&E section showing a classical MTC (m) growing in solid nests infiltrating adjacent non-neoplastic thyroid (thy) (100x). b Immunostain for calcitonin on the same tissue section present in A confirming the MTC diagnosis (100x). Tumor is immunopositive for calcitonin (m) while the non-neoplastic thyroid is negative (thy) (100x). c H&E from another area of the primary tumor showing fresh tumor necrosis (n) surrounded by viable tumor (m) (100x). d High power view of an H&E from another focus of tumor necrosis (n) showing nuclear debris (arrow). This appearance of the necrosis is referred to as “comedo-like”. The viable tumor cells (m) display no significant nuclear pleomorphism (200x).
Patient was M0 at presentation with postoperative calcitonin and CEA of 124 pg/ml and 20.4 ng/ml, respectively. The patient died of disease 4 years and 4 months after initial surgery. a Medium power view of an H&E section from the primary showing a classical MTC (m) growing in solid nests adjacent to non-neoplastic thyroid (thy) (100x). b Medium power view of an H&E section from a regional node with metastatic tumor at presentation. There is fresh tumor necrosis (n) surrounded by viable tumor (m) (100x). c Higher power view of the necrotic area in B showing tumor necrosis (n) containing nuclear debris (arrow). The viable tumor cells (m) do not show significant nuclear atypia (200x). d Immunostain for calcitonin on the same tissue section present in B confirming the MTC diagnosis. The viable tumor surrounding the necrotic focus (n) is immunopositive for calcitonin (m) (100x).
Clinical and biochemical parameters
Clinical, biochemical, and follow-up data were obtained by review of the medical records. The following parameters were documented: Age at thyroidectomy, sex, germline RET status, type of thyroid surgery, postoperative serum calcitonin and CEA levels (defined as the first postoperative level within 30 days of surgery) and distant metastatic status at presentation and on follow up. Locoregional recurrence was defined as structural recurrence in the neck noted on imaging studies while distant recurrence was defined on the basis of biochemical and radiographic evidence of structural disease outside of the neck with or without biopsy. Patients with increased calcitonin without structural correlate were not included in the distant metastasis classification. Locoregional and distant recurrences were recorded independently and the time to recurrence or death was calculated from the date of surgery. The patients’ stage at presentation was assessed using the 8th edition of the AJCC staging system [15].
Statistical analysis
All statistical analyses were performed using the SPSS software 24.0 (IBM Corporation, New York, NY, USA). Tumor size was correlated with the presence/absence of tumor necrosis using two-tailed Student’s t test. The prognostic significance of each parameter on disease specific survival (DSS), loco-regional recurrence free survival (LRFS), and distant metastasis free survival (DMFS) was calculated using univariate Cox proportional model for log-transformed postoperative CEA and calcitonin levels and log rank tests for all other variables. Factors significant on univariate analysis were subsequently subjected to multivariate analysis using Cox proportional hazards model. Factors that were only applicable to a subset of patients (e.g., the characteristics of LVI and nodal metastasis) were excluded from the multivariate analysis. P values less than 0.05 were considered to be statistically significant.
Results
Clinico-pathologic characteristics of the study cohort
The clinico-pathologic features of the 144 patients are reported in Table 1. The female to male ratio was ~1:1. The median age at diagnosis was 53 years (range: 3–88 years). The median tumor size was 1.8 cm (range: 0.2–11 cm). Four (3%) were treated with lobectomy alone, while the remaining 140 (97%) underwent total thyroidectomy.
Mitosis was present in 52 (36%) cases while absent in 92 (64%) of patients. In those 52 mitotically active tumors, the median number of mitoses was 2/10 high power fields (HPFs, range: 1–20) and atypical (abnormal) mitoses were found in 20 (38%) of mitotically active carcinomas. Tumor necrosis was present in 30 (21%) of patients. Patients with tumor necrosis had larger carcinomas (median 3.0 cm) than those without necrosis (median 2.1 cm, p < 0.001). The vast majority of cases (87%) displayed none/mild nuclear pleomorphism. A significant (moderate/marked) amount of intratumoral fibrosis was seen in 76% of MTC. A minority of carcinomas (n = 26, 18%) were completely encapsulated/well circumscribed. Sixteen (62%) of these 26 totally encapsulated/well circumscribed tumors revealed capsular invasion and 7 (27%) harbored LVI. In eight patients, the dominant tumor was completely encapsulated/well circumscribed and lacked any invasion. These noninvasive tumors comprised 31% of the encapsulated/well circumscribed carcinoma and 6% of the entire study population. Overall, LVI was found in 41 (29%) of individuals. In patients with LVI, extra-thyroidal LVI was seen in 20 (49%) of cases. Extra-thyroidal extension and positive margins were present in 31 and 14% of cases, respectively. In patients with extra-thyroidal extension, invasion was seen in perithyroidal fibroadipose tissue in 39/44 (89%), in skeletal muscle in 4/44 (9%) and in trachea in 1/44 (2%).
At presentation, 84 (58%) of all patients had nodal disease and 9 (6%) harbored distant metastasis. In patients with nodal metastasis, the median number of positive nodes was 7 (range: 1–60). The median size of the largest nodal metastasis was 1.6 cm (range: 0.01–9.5 cm).
Among the 136 patients with available germline RET mutation analysis, 28 (21%) had familial MTC while 106 (79%) had sporadic tumors. Among the 22 cases with mutation details available, the affected codons were 618 (n = 2), 620 (n = 2), 634 (n = 8), 639 (n = 1), 790 (n = 2), 791 (n = 2, including one Y791F), 804 (n = 2), and 918 (n = 3). The median postoperative serum calcitonin was 43 pg/ml (range: 0–970000 pg/ml). In regard to postoperative serum CEA, the median was 5.6 ng/ml (range: 0.5–1889 ng/ml).
Survival analysis
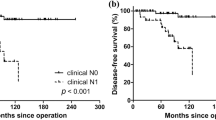
With a median follow up of 39 months (range: 0.3–297 months), the 5-year OS and DSS were 87% and 94%, respectively. The 5-year LRFS and DMFS were 77% and 87%, respectively. Table 2 shows the p values of univariate survival analysis. Male sex, large tumor size, mitotic index of 5 or more mitoses/10 HPFs, the presence of tumor necrosis, nodal metastasis, AJCC stage grouping, large size of largest lymph node metastatic focus and higher postoperative serum calcitonin levels imparted worse DSS, LRFS, and DMFS (Fig. 3). An increase in mitotic activity subdivided into three categories (<2, 2–10, and >10 mitoses/10 HPFs) was associated with poorer DSS, LRFS, and DMFS. Marked nuclear pleomorphism imparted worse DSS, LRFS but not DMFS. Tumor encapsulation improved LRFS but had no effect on DSS and DMFS. Infiltration was associated with shortened LRFS. Neither the number of positive nodes nor extra-nodal extension impacted survival. Patients with extrathyroidal extension had poorer DSS and DMFS with no effect on LRFS. Positive margins correlated with worse DSS only. The presence of LVI imparted worse DSS only while extensive LVI correlated solely with worse DMFS. Distant metastasis at presentation correlated with poorer DSS. Age, familial disease, tumor multifocality, degree of intra-tumoral fibrosis, atypical mitosis, the presence of amyloid and postoperative serum CEA did not significantly impact any outcome measure.
The results of subsequent multivariate survival analysis are shown in Table 3. Factors significant on univariant analysis were entered in a multivariate analysis, with the exception of size of largest nodal metastasis (as it would limit the cases to only those with N1 disease only) and nodal metastasis/tumor size/DM at presentation as they overlapped with the AJCC stage group. Tumor necrosis and mitotic activity (using 5 mitoses/10 HPFs as the cutoff) were the only independent predictors for DSS (p = 0.004 and 0.020, respectively). A cutoff of 2 and 10 mitoses per 10 HPFs did not reach significance level on multivariate analysis (p = 0.388). Tumor necrosis was the only independent prognostic factor for LRFS and DMFS (p = 0.001 and 0.003, respectively). Other factors, including sex, AJCC stage group, infiltration, encapsulation, nuclear pleomorphism, extrathyroidal extension, margin status, and/or postoperative calcitonin level did not independently predict survival (p > 0.05). Mitotic levels (both thresholds) did not independently predict LRFS and DMFS (p > 0.05).
Proposed grading system and its prognostic significance
Based on the results of the survival analysis, we herein proposed a two-tiered grading system of MTC based on mitosis and necrosis (Table 4). An MTC could be considered as low-grade if it contained <5 mitosis/10 HPF and no tumor necrosis, and as high grade if it has ≥5 mitosis/10 HPF and/or tumor necrosis. Using this grading system, 31 MTCs (22%) were classified as high grade, whereas the remaining 113 (78%) were considered as low grade. Univariate survival analysis using log rank test showed that this grading system predicted DSS (p < 0.001, Fig. 3c), LRFS (p < 0.001), and DMFS (p < 0.001). Multivariate analysis using Cox proportional model including the proposed grading scheme and the AJCC stage group showed that both grade and stage were independent prognostic factors for LRFS (stage: p = 0.029, hazard ratio = 1.548, 95% confidence interval: 1.045–2.293; grade: p < 0.001, hazard ratio = 5.142, 95% confidence interval 2.367–11.170), and DMFS (stage: p = 0.014, hazard ratio = 2.174, 95% confidence interval: 1.171–4.037; grade: p < 0.001, hazard ratio = 6.304, 95% confidence interval 2.310–17.201), whereas grade but not stage was an independent prognostic factor for DSS (grade: p = 0.001, hazard ratio = 10.546, 95% confidence interval 2.782–39.977). We also used the three-tiered grading system utilized in pulmonary NET to grade MTC. This system is based on necrosis and a three-tiered mitotic index (cutoff of 2 and 10 mitosis/2 mm2) [16]. Although the pulmonary NET grading scheme was overall significant in univariate and multivariate analysis when adjusted for stage grouping (OS: p < 0.001, DSS: p = 0.001, LRFS: p < 0.001, and DMFS: p < 0.001), there were only two cases classified as grade 3 (equivalent to pulmonary neuroendocrine carcinomas, >10 mitosis/2 mm2), and their survival did not differ from grade 1, whereas the grade 2 MTC were associated with the worst prognosis (Supplementary Fig. 1).
Discussion
The rarity of MTC and the broad spectrum of its clinical behavior renders prediction of outcome a difficult task [1, 9]. A set of clinico-pathologic features have been commonly used to prognosticate these tumors and develop staging systems. By univariate analysis, tumor size, age, male sex, extrathyroidal extension, extent of thyroidectomy, lymph node metastases, distant metastases at presentation, serum calcitonin, CEA blood levels, the presence of familial disease and somatic RET mutation status have been shown to impact survival [1, 4,5,6,7, 15, 17,18,19]. However, in multivariate analysis, only age and stage at presentation remain as independent prognostic variables in most publications [1, 4, 18]. In congruence with the above studies, we found that male sex, large tumor size, the presence of nodal disease, distant metastases at presentation, stage, elevated postoperative serum calcitonin and extra-thyroidal extension imparted worse survival in univariate analysis. In contrast, older age did not correlate with death or disease recurrence in our cohort. This discrepancy in regard to the prognostic value of age is well known. Indeed, several studies have shown that age does not influence survival in MTC [9, 20, 21]. That could be the reason behind the current AJCC system not using age as a staging parameter in MTC.
Many authors have attempted to find histologic markers of outcome in this unpredictable disease. As early as 1966, Williams et al. reported an association between a predominant spindle cell pattern, mitosis, necrosis, and poorer survival in a series of 67 MTC [22]. However, no formal statistical analysis was performed by these authors. Subsequent studies have not shown any prognostic value for cell type, dominant architecture, multicentricity, or amyloid deposition [23,24,25]. As shown in Table 2, we confirm the lack of prognostic significance of multicentricity and amyloid deposition.
In 2008, Koperek et al. found a statistically significant correlation between the presence of a desmoplastic stroma and nodal metastasis [26]. In contrast, we and others [25, 27, 28] did not find any prognostic value for the presence or the degree of fibrosis in MTC. One possible explanation is the interobserver variability inherent to the evaluation of such a subjective histologic parameter.
In the current series, completely encapsulated and noninfiltrative MTC imparted a statistically better LRFS on univariate analysis. These results are in accordance with the study of Miccoli et al. [27]. In an analysis of 70 MTC, these authors found that the presence of a complete tumor capsule and lack of infiltration correlate strongly with a high biochemical cure rate and especially an absence of nodal metastasis [27]. Therefore, they proposed to use tumor encapsulation and thyroid parenchyma infiltration as tools to stratify patients for cervical lymph node dissection in MTC [27].
The presence of LVI was associated with worse DSS in our patient cohort, and extensive (≥4 foci) LVI with worse DMFS on univariate analysis. This is in congruence with several studies that found a positive correlation between the sole presence of vascular invasion and recurrence and death in MTC [26, 29, 30]. While Rios et al. found that the presence of vascular invasion is an independent predictor of recurrence, it lost its predictive value when analyzed in multivariate analysis in the study herein and by others [29]. These disparate results could in part be due to differences in the criteria used to diagnose vascular invasion. While we characterized vascular invasion as a tumor thrombus hanging in the lumen of a vessel located in the thyroid or extrathyroidal soft tissue, Pilaete et al. describe vascular invasion as “histologic vascular invasion in thyroid specimen or metastatic lymph node” [30]. The publication of Rios et al. defines vascular invasion as “tumor cells inside or on the vessel walls” without mention of the location of the involved vessels [25]. Clearly, additional studies using more uniform criteria for vascular invasion are needed in MTC.
Although nuclear pleomorphism is used to grade many solid tumors, we found very few publications on the subject in MTC. Skopelitou et al. found a correlation between nuclear grade (defined in their article by a combination of nuclear pleomorphism and mitosis) and the proliferative marker Proliferating Cell Nuclear Antigen detected by immunostaining [31]. They however did not analyze nuclear pleomorphism on its own and did not assess its effect on survival. In our patient population, nuclear pleomorphism imparted worse DSS and LRFS but not DMFS while Williams et al. did not find such a correlation [22]. There could be many explanations for these disparate findings. The survival impact of nuclear pleomorphism could be confounded by the fact that some MTC display spotty marked nuclear atypia not associated with mitosis (so called endocrine atypia). This phenomenon found in many benign and malignant endocrine and NET can be alarming under the microscope but is of no clinical significance [32]. Nuclear pleomorphism also suffers from being quite a subjective microscopic feature amenable to significant interobserver variability. For the above reasons, it is our opinion that nuclear pleomorphism is not a robust tool to predict behavior in MTC.
The data on the predictive value of mitosis and necrosis in MTC is controversial. While most studies performed in the last two decades have found necrosis to be of prognostic value in univariate analysis [24, 33, 34], only Franc et al. showed this feature to be an independent predictor of disease-specific mortality [34]. We confirm that tumor necrosis is an independent prognosticator of DSS and expand its independent predictive value to LRFS and DMFS. The reason for these discrepant results could reside in the size of the study cohorts. Indeed, the publications showing independent predictive value have a larger patient population (n = 144 for our study and n = 109 for the one of Franc et al.) than the one of Dottorini et al. (n = 53) which did not find necrosis to be significant in multivariate analysis [10]. Another explanation could be the smaller rate of patients with tumor necrosis in the paper of Dottorini et al. (7.5%) compared with the study herein (21%) and the one of Franc et al. (50%).
In regard to mitosis, the data is scant. There is only one published recent paper that analyzed mitosis using appropriate statistics for survival analysis [33]. These authors did not find any survival difference based on mitosis by univariate analysis. In contrast we found mitosis to be an independent predictor of DSS. These divergent results could be due to a difference in cut off for mitotic count. In the study of Rios et al. [33], a high mitotic index was described as “mitotic figures predominant in the tumor” while we used a cut off of five or more mitosis per ten HPFs to define high mitotic activity. Whatever the reason for the incongruities in the study of mitosis and necrosis and other histologic variables, this uncertainty led the authors of the most recent AJCC not to incorporate these histologic features in their staging system [15].
To the best of our knowledge, this is the first study that shows that mitosis and tumor necrosis are both independent predictors of poor outcome in MTC. The latter is a particularly strong indicator of worse survival since necrosis was an independent prognostic variable for each of DSS, LRFS, and DMFS in this cohort. In our analysis, tumor necrosis was found to be independent from established prognostic factors such as sex, extra-thyroidal extension, AJCC stage and postoperative serum calcitonin making it quite valuable and powerful. In addition, mitosis and tumor necrosis are relatively well-defined and objective histologic features less prone to interobserver variability than for example vascular invasion or cell shape. However, the identification of these parameters still requires the skills of a well-trained pathologist. Indeed, it is important to distinguish tumor necrosis from necrosis related to fine needle aspiration. Tumor necrosis is composed of degenerating cytoplasm and punctuate, karyorrhectic nuclear debris (so called “comedo-like” necrosis) (Figs. 1, 2). It lacks the fibroblastic stromal reaction, hemorrhage or identifiable needle track found in fine needle aspiration induced necrosis [35].
The fact that mitosis and tumor necrosis are so powerful in predicting outcome in MTC should not come as a surprise. The presence of mitosis and/or tumor necrosis is utilized to grade other neuroendocrine and endocrine tumors such as those from the lung and pancreas [12, 13]. Armed with that knowledge, we attempted to use the mitotic rate cut off that stratify NET of the lung into three separate prognostically different categories (<2, 2–10, >10 mitoses/10 HPFs, ≈2 mm2) [12]. Although this three-tiered mitotic system correlated with survival in univariate analysis, it was not an independent predictor of outcome. We also graded MTC using the three-tiered grading scheme utilized in pulmonary NET (tumor necrosis and the three-tiered mitotic index described above). While the pulmonary grading system was overall significant in univariate and even multivariate analysis when adjusted for stage, there were only two cases classified as grade 3 (equivalent to pulmonary neuroendocrine carcinomas), and their survival did not differ from grade 1, whereas the grade 2 MTC were associated with the worse prognosis. This data suggests that the three-tiered grading system of pulmonary NET is impractical for MTC. In view of the latter fact together with the apparent superior predictive value of the two-tiered mitotic index cutoff and its simplicity over a three-tiered mitotic rate grouping, we chose the prognostically significant two tiered cut off at 5 mitoses/10 HPFs to construct our proposed grading system. Interestingly, the same mitotic count cut off can define poorly differentiated thyroid carcinomas of follicular cell origin and separate them from their better differentiated counterparts [36].
Since mitosis seems prognostically important in MTC and NET in general, one would assume that the proliferative marker ki-67 will be a helpful tool to predict behavior. For example, the current grading system for pancreatic neuroendocrine neoplasms also include Ki-67 proliferation index as a component of the grading system with a three-tiered cutoff of 2 and 20% [13]. In a series of 36 patients with MTC, Tinsel et al. showed a strong correlation between the percentage of Ki-67 immunopositive tumor cells and outcome using a semiautomatic image analysis program [37]. However, the ki-67 indices of the primary tumors were overall quite low (median 0.41%, range: 0.01–4.5%) making this marker difficult to use in everyday practice and a three-tiered cutoff of 2 and 20% akin to what has been used for pancreatic neuroendocrine neoplasms impractical for MTC [37]. There is a clear need to further investigate ki-67 and especially phosphohistone H3 (an immunohistochemical marker specific for mitotically active cells) [38] in MTC.
This study has several limitations. First, it is retrospective in nature. This is however almost always the rule in a rare disease such as MTC. Second, we did not evaluate the effect of somatic RET or RAS mutation on prognosis since some of our patients were operated in outside institutions making the retrieval of their tissue difficult. In that regard some authors have found that somatic RET mutation status correlated with worse outcome [7, 39] while others did not find such a relationship [40, 41]. In a study of 51 sporadic MTC cases, Moura et al. divided their patients into three groups: those with RET mutations in exons 15 and 16, cases with other RET mutations and patients who were wild type for RET. The group with RET mutations in exons 15 and 16 had a higher prevalence and number of nodal metastasis than those with other RET mutations [40]. There were however no statistically significant survival differences between the three groups of patients [40]. Ciampi et al. found a correlation between RAS mutation and better outcome in MTC but it did not reach statistical significance [42]. Although the data in the literature is overall encouraging [43], larger studies using multivariate analysis are needed to assess the additive prognostic value of molecular profiling in sporadic MTC.
Despite these limitations, this study has many advantages. It was performed on a large series of patients (n = 144) followed in the same institution. It consisted of a very meticulous and especially detailed histopathologic analysis. Recurrence was defined as structural recurrence and therefore as a clinically relevant outcome measure. It demonstrated that mitosis and necrosis are very powerful indicators of poor survival in MTC independent from well-established prognostic factors such as AJCC stage at presentation and postoperative serum calcitonin. In the clinical setting, the American Thyroid Association (ATA) guidelines state that “TNM classification and other factors, such as the postoperative calcitonin level and the calcitonin and CEA doubling times, should be used to predict outcome and to help plan long-term follow-up of patients with MTC” [1]. In the opinion of the ATA panel, stage lacks important prognostic factors such as calcitonin and CEA doubling times [1]. However, these latter parameters change over time, being longer when the disease is in its early stages and shorter in later stages, when there is disease progression [42, 44]. The grading of MTC based on mitosis and necrosis will hopefully pave the way to a customized patient follow-up and therapy from diagnosis. Herein, we propose to grade MTC into low grade (<5 mitosis/10 HPF and no tumor necrosis) and high grade (≥5 mitosis/10 HPF and/or tumor necrosis, Table 4). This grading system is independent from stage in predicting LRFS and DMFS and the sole independent prognostic factor for DSS in our cohort. This will enable the treating clinician to better counsel the patient, design the optimal follow up and better select those individuals who may benefit from systemic therapy.
References
Wells SA Jr., Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567–610.
Williams ED. Histogenesis of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:114–8.
Tashjian AH Jr., Melvin EW. Medullary carcinoma of the thyroid gland. Studies of thyrocalcitonin in plasma and tumor extracts. N Engl J Med. 1968;279:279–83.
Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer. 2000;88:1139–48.
Opsahl EM, Akslen LA, Schlichting E, Aas T, Brauckhoff K, Hagen AI, et al. Trends in diagnostics, surgical treatment, and prognostic factors for outcomes in medullary thyroid carcinoma in Norway: a nationwide population-based study. Eur Thyroid J. 2019;8:31–40.
Turkdogan S, Forest VI, Hier MP, Tamilia M, Florea A, Payne RJ. Carcinoembryonic antigen levels correlated with advanced disease in medullary thyroid cancer. J Otolaryngol Head Neck Surg. 2018;47:55.
Elisei R, Cosci B, Romei C, Bottici V, Renzini G, Molinaro E, et al. Prognostic significance of somatic RET oncogene mutations in sporadic medullary thyroid cancer: a 10-year follow-up study. J Clin Endocrinol Metab. 2008;93:682–7.
Lindsey SC, Ganly I, Palmer F, Tuttle RM. Response to initial therapy predicts clinical outcomes in medullary thyroid cancer. Thyroid. 2015;25:242–9.
Hassan A, Siddique M, Riaz S, Khan AI, Nawaz MK, Bashir H. Medullary thyroid carcinoma: prognostic variables and tumour markers affecting survival. J Ayub Med Coll Abbottabad. 2018;30 Suppl 1:S627–32.
Hazard JB, Hawk WA, Crile G Jr. Medullary (solid) carcinoma of the thyroid; a clinicopathologic entity. J Clin Endocrinol Metab. 1959;19:152–61.
Jaquet J. Ein Fall von metastasierenden Amyloidtumoren (Lymphosarkom). Virchows Arch Pathol Anat Physiol. 1906;185:251–68.
D. TW, Brambilla E, Burke AP, A. M, G. NA. WHO classification of tumours of the lung pleura, thymus and heart. Lyon: International Agency for Research on Cancer; 2015.
Lloyd RV, Osamura RY, Kloppel G, Rosai J. WHO classification of tumours of endocrine organs. Lyon: International Agency for Research on Cancer (IARC); 2017.
Rosai J, DeLellis RA, Carcangiu ML, Frable WJ, Tallini G. Tumor of the thyroid and parathyroid gland (AFIP atlas of tumor pathology series 4). Silver Spring, MD: American Registry of Pathology Press; 2015. p. 606.
Amin MB, Edge SB, L. GF, Brookland RK, M. K, E. GJ, et al. AJCC cancer staging manual. 8th edition. Chicago, IL: Springer Nature; 2017.
Hendifar AE, Marchevsky AM, Tuli R. Neuroendocrine tumors of the lung: current challenges and advances in the diagnosis and management of well-differentiated disease. J Thorac Oncol. 2017;12:425–36.
Ito Y, Miyauchi A, Kihara M, Higashiiyama T, Fukushima M, Miya A. Static prognostic factors and appropriate surgical designs for patients with medullary thyroid carcinoma: the second report from a single-institution study in Japan. World J Surg. 2018;42:3954–66.
Modigliani E, Cohen R, Campos JM, Conte-Devolx B, Maes B, Boneu A, et al. Prognostic factors for survival and for biochemical cure in medullary thyroid carcinoma: results in 899 patients. The GETC Study Group. Groupe d'etude des tumeurs a calcitonine. Clin Endocrinol. 1998;48:265–73.
Ho AS, Wang L, Palmer FL, Yu C, Toset A, Patel S, et al. Postoperative nomogram for predicting cancer-specific mortality in medullary thyroid cancer. Ann Surg Oncol. 2015;22:2700–6.
Brandao LG, Cavalheiro BG, Junqueira CR. Prognostic influence of clinical and pathological factors in medullary thyroid carcinoma: a study of 53 cases. Clinics. 2009;64:849–56.
Gulben K, Berberoglu U, Boyabatli M. Prognostic factors for sporadic medullary thyroid carcinoma. World J Surg. 2006;30:84–90.
Williams ED, Brown CL, Doniach I. Pathological and clinical findings in a series of 67 cases of medullary carcinoma of the thyroid. J Clin Pathol. 1966;19:103–13.
Schroder S, Bocker W, Baisch H, Burk CG, Arps H, Meiners I, et al. Prognostic factors in medullary thyroid carcinomas. Survival in relation to age, sex, stage, histology, immunocytochemistry, and DNA content. Cancer. 1988;61:806–16.
Dottorini ME, Assi A, Sironi M, Sangalli G, Spreafico G, Colombo L. Multivariate analysis of patients with medullary thyroid carcinoma. Prognostic significance and impact on treatment of clinical and pathologic variables. Cancer. 1996;77:1556–65.
Rios A, Rodriguez JM, Acosta JM, Balsalobre MD, Torregrosa N, Sola J, et al. Prognostic value of histological and immunohistochemical characteristics for predicting the recurrence of medullary thyroid carcinoma. Ann Surg Oncol. 2010;17:2444–51.
Koperek O, Scheuba C, Cherenko M, Neuhold N, De Micco C, Schmid KW, et al. Desmoplasia in medullary thyroid carcinoma: a reliable indicator of metastatic potential. Histopathology. 2008;52:623–30.
Miccoli P, Minuto MN, Ugolini C, Molinaro E, Basolo F, Berti P, et al. Clinically unpredictable prognostic factors in the outcome of medullary thyroid cancer. Endocr Relat Cancer. 2007;14:1099–105.
Rougier P, Parmentier C, Laplanche A, Lefevre M, Travagli JP, Caillou B, et al. Medullary thyroid carcinoma: prognostic factors and treatment. Int J Radiat Oncol Biol Phys. 1983;9:161–9.
Brierley J, Tsang R, Simpson WJ, Gospodarowicz M, Sutcliffe S, Panzarella T. Medullary thyroid cancer: analyses of survival and prognostic factors and the role of radiation therapy in local control. Thyroid. 1996;6:305–10.
Pilaete K, Delaere P, Decallonne B, Bex M, Hauben E, Nuyts S, et al. Medullary thyroid cancer: prognostic factors for survival and recurrence, recommendations for the extent of lymph node dissection and for surgical therapy in recurrent disease. B-ENT. 2012;8:113–21.
Skopelitou A, Korkolopoulou P, Papanikolaou A, Hadjiyannakis M. Proliferating cell nuclear antigen (PCNA) in medullary thyroid carcinoma. J Cancer Res Clin Oncol. 1993;119:379–81.
DeLellis RA. Parathyroid tumors and related disorders. Mod Pathol. 2011;24 (Suppl 2):S78–93.
Raue F, Kotzerke J, Reinwein D, Schroder S, Roher HD, Deckart H, et al. Prognostic factors in medullary thyroid carcinoma: evaluation of 741 patients from the German Medullary Thyroid Carcinoma Register. Clin Investig. 1993;71:7–12.
Franc B, Rosenberg-Bourgin M, Caillou B, Dutrieux-Berger N, Floquet J, Houcke-Lecomte M, et al. Medullary thyroid carcinoma: search for histological predictors of survival (109 proband cases analysis). Hum Pathol. 1998;29:1078–84.
Rivera M, Ricarte-Filho J, Patel S, Tuttle M, Shaha A, Shah JP, et al. Encapsulated thyroid tumors of follicular cell origin with high grade features (high mitotic rate/tumor necrosis): a clinicopathologic and molecular study. Hum Pathol. 2010;41:172–80.
Hiltzik D, Carlson DL, Tuttle RM, Chuai S, Ishill N, Shaha A, et al. Poorly differentiated thyroid carcinomas defined on the basis of mitosis and necrosis: a clinicopathologic study of 58 patients. Cancer. 2006;106:1286–95.
Tisell LE, Oden A, Muth A, Altiparmak G, Molne J, Ahlman H, et al. The Ki67 index a prognostic marker in medullary thyroid carcinoma. Br J Cancer. 2003;89:2093–7.
Kim YJ, Ketter R, Steudel WI, Feiden W. Prognostic significance of the mitotic index using the mitosis marker anti-phosphohistone H3 in meningiomas. Am J Clin Pathol. 2007;128:118–25.
Mian C, Pennelli G, Barollo S, Cavedon E, Nacamulli D, Vianello F, et al. Combined RET and Ki-67 assessment in sporadic medullary thyroid carcinoma: a useful tool for patient risk stratification. Eur J Endocrinol. 2011;164:971–6.
Moura MM, Cavaco BM, Pinto AE, Domingues R, Santos JR, Cid MO, et al. Correlation of RET somatic mutations with clinicopathological features in sporadic medullary thyroid carcinomas. Br J Cancer. 2009;100:1777–83.
Simbolo M, Mian C, Barollo S, Fassan M, Mafficini A, Neves D, et al. High-throughput mutation profiling improves diagnostic stratification of sporadic medullary thyroid carcinomas. Virchows Arch. 2014;465:73–8.
Ciampi R, Mian C, Fugazzola L, Cosci B, Romei C, Barollo S, et al. Evidence of a low prevalence of RAS mutations in a large medullary thyroid cancer series. Thyroid. 2013;23:50–7.
Fussey JM, Vaidya B, Kim D, Clark J, Ellard S, Smith JA. The role of molecular genetics in the clinical management of sporadic medullary thyroid carcinoma: a systematic review. Clin Endocrinol. 2019;91:697–707.
Cavedon E, Barollo S, Bertazza L, Pennelli G, Galuppini F, Watutantrige-Fernando S, et al. Prognostic impact of miR-224 and RAS mutations in medullary thyroid carcinoma. Int J Endocrinol. 2017;2017:4915736.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No competing financial interests exist for all contributory authors. Research reported in this publication was supported in part by the Cancer Center Support Grant of the National Institutes of Health/National Cancer Institute under award number P30CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Alzumaili, B., Xu, B., Spanheimer, P.M. et al. Grading of medullary thyroid carcinoma on the basis of tumor necrosis and high mitotic rate is an independent predictor of poor outcome. Mod Pathol 33, 1690–1701 (2020). https://doi.org/10.1038/s41379-020-0532-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-020-0532-1
This article is cited by
-
International medullary thyroid carcinoma grading system: an Indian tertiary care centre experience
European Archives of Oto-Rhino-Laryngology (2024)
-
Diagnostic, Prognostic, and Predictive Role of Ki67 Proliferative Index in Neuroendocrine and Endocrine Neoplasms: Past, Present, and Future
Endocrine Pathology (2023)
-
Valutazione del grading nel carcinoma midollare tiroideo
L'Endocrinologo (2023)
-
Exploration of Digital Image Analysis for Ki67 Quantification in the Grading of Medullary Thyroid Carcinoma: A Pilot Study with 85 Cases
Head and Neck Pathology (2023)
-
The Impact of the 2022 WHO Classification of Thyroid Neoplasms on Everyday Practice of Cytopathology
Endocrine Pathology (2023)