Abstract
Although all diagnoses in dermatopathology are important, three main groups may be highlighted. One group includes diagnoses that need to be communicated to the treating physician as soon as possible (this review includes infectious process while erythema multiforme and related diseases are discussed elsewhere in this series). A second group has diagnoses significant for their association with syndromes or internal malignancies. And a third group includes malignant lesions that can be confused histologically with benign ones or lesions that have an aggressive behavior unexpected for their apparently low-grade histology. This manuscript describes some of these important diseases and the method we use to reach the diagnosis, and as such it may be considered to be a “survival” guide for the dermatopathologist.
Similar content being viewed by others
Introduction
One of the most important ways which may help detect these possible pitfalls is the use of a systematic approach to the diagnosis [1]. As stressed by one of our mentors, Dr. Scott McNutt, it is advisable to be “compulsive” when reviewing slides. He meant by it to follow a routine to minimize the possibility of “missing” something. For example, he would always place the slide on the stage (or as he would say nowadays, with the digital image handling software) with the epidermis on top. Then he would look at the entire slide in a systematic manner from top to bottom, paying attention to possible changes present in the stratum corneum, stratum granulosum, stratum spinosum, basal layer, basement membrane area, papillary dermis, reticular dermis, and subcutaneous tissue. Since some of the changes may be subtle (such as tinea nigra organisms in the stratum corneum), by using the systematic approach it will be easier to detect those subtle changes if we always look into the stratum corneum. Another advantage of such systematic approach is the possible detection of more than one finding in the slide. Thus, if a skin biopsy has a seborrheic keratosis in the epidermis and our brain focuses just on that area, we may ignore the rest of the specimen, and then we might miss the presence of metastatic melanoma in the dermal vessels!
Another component of the systematic approach refers to the “right” moment to correlate the histologic findings with any other available information, such as clinical data, prior biopsies, etc. Again as Dr. Scott McNutt recommended to us, many dermatopathologists prefer to review the slide without knowledge of any clinical diagnosis. Although this approach may not be the preferred one by general surgical pathologists, it is our opinion that it allows to keep an open mind when reviewing the slides. If we know that the clinician wants to rule out a pigmented lesion, we may concentrate our analysis only to the superficial regions of the skin (epidermis and upper dermis, but then miss the presence of leukemia cutis). Similarly, if the clinical diagnosis is “nevus” but we are not informed that the patient has a prior history of metastatic melanoma, we may interpret a melanocytic lesion lacking obvious intraepidermal extension to be benign (i.e., nevus) and thus ignore the possibility of a melanoma metastatic to the dermis. At any rate, it goes without saying that the FINAL report should include ALL available information (clinical, serological, radiological, results of special studies), and not only that detected on the slide.
On the other hand, a possible negative result of examining the slides without clinical information and discussing such preliminary diagnosis with a colleague, resident or fellow, may be that of suffering a diagnostic “blunder” with the subsequent ego “bruising”. As an example, a lesion on the cheek in a 70-year-old woman with atrophy of the epidermis, vacuolated cells at the dermal epidermal junction, “consumption” of the epidermis (also known as “zipper” sign (personal communication, Shea CR)), and presence of pigmented cells in the papillary dermis may be considered to be lentigo maligna type of melanoma in situ with regression until the clinical history is reviewed to indicate that the biopsy is from a bilateral plaque in an African American woman, and thus likely corresponds to a discoid lesion of lupus erythematosus!
This chapter reflects on how we apply the systematic method to a selected group of dermatopathology diseases.
Dermatopathologic emergencies/Infectious diseases
There are several infectious diseases that affect the skin and require expedite management of the patient due to their severity.
Among the fungal diseases, infection by Mucor, Fusarium, and Aspergillus are probably the most frequent and important in our country [2].
Mucor sp. is an aerobic saprophyte that can be found almost in every continent. It particularly affects immunosuppressed individuals, including oncologic and diabetic patients. The most common locations are the nose, lung, gastrointestinal tract, and skin. Clinically it presents with small, erythematous macules that grow into nodules and may ulcerate with a black eschar. Histologically, the dermis and subcutis are usually involved with areas of necrosis and relatively little inflammatory infiltrate (small and scattered foci of neutrophils and macrophages). In the areas of necrosis, sometimes within vessels, and when using a ×40 objective, there will be broad (10−20 μm in diameter), nonseptate hyphae with branching close to 90° (Fig. 1) [3]. Due to the presence of angioinvasion there may be frank vasculitis, thrombosis, hemorrhage and ischemic infarction. In rare cases the organisms may affect the epidermis. Special stains (Grocott methenamine stain, GMS) or immunohistochemistry will highlight the organisms (Fig. 1). Treatment of Mucor requires immediate antifungal therapy and, in many occasions, surgical resection of the affected area.
A less known form of Mucor infection, commonly caused by Mucor irregularis, may present with chronic lesions, mostly in immunocompetent individuals [4].
Fusarium sp. tends to present as cutaneous lesions, mostly in neutropenic patients with underlying hematological malignancies. When left untreated it disseminates hematogenously and reaches up to 80% mortality. Thus, it requires immediate antifungal treatment. Histologically, and similar to Mucor, there is relatively little inflammation, with areas of necrosis and small foci of neutrophils and macrophages in dermis and subcutis. The organisms are smaller than Mucor, with septate hyphae between 3 and 8 μm in diameter, and irregular branching ranging from 30 to 90° (Fig. 2). There are large macroconidia that resemble “lollypops”, as highlighted by GMS (Fig. 2) [5].
Aspergillus sp. affects mostly immunosuppressed patients, particularly those treated with chronic corticosteroids. It spreads to the skin through the blood or else as direct cutaneous inoculation. It can be acquired from air condition vents and central heating systems. Histologically there are zones of necrosis with small foci of septate hyphae around 4 μm in diameter, with regular branching of 45° (Fig. 3). When numerous enough, the organisms are arranged in tightly packed clusters of hyphae. GMS and immunohistochemistry will help detect the fungi (Fig. 3). As with Mucor, some cases may require surgery in addition to antifungal therapy [6].
There are several viral processes that are relatively frequently diagnosed in cutaneous biopsies.
Herpes zoster or simplex typically involve the epithelium (epidermis, adnexa, or mucosae). There are grouped vesicles, many times in a dermatomal distribution. The hallmark is the presence of epithelial multinucleated giant cells with molding of the nuclei (Fig. 4). These cells may be associated with epithelial necrosis and acantholysis. Involvement of the pilosebaceous units can be seen especially in recurrent lesions, with the infected cells usually located in the outer root sheath. Herpetic syringitis refers to involvement of the eccrine ducts [7, 8]. Herpes simplex more frequently involves the epithelium (commonly hair follicles and eccrine glands) while zoster tends to reveal a denser perivascular infiltrate (personal observation). However, definite genotyping requires immunohistochemistry (Fig. 4).
Epstein Barr virus traditionally has been detected in some lymphoproliferative disorders such as mononucleosis, which rarely involve the skin. However, there are cases of cutaneous lesions in immunosuppressed patients, such as those receiving solid organ transplantation, that are designated as a group of post-transplant lymphoproliferative disorders, ranging from reactive to fully neoplastic (see below). Similarly, angioimmunoblastic lymphoma and CD8-positive T-cell lymphoma typically show expression of EBV in the neoplastic cells. Finally, there are some solitary EBV lesions, mostly in elderly patients, that present as cutaneous ulcers.
Bacterial infections
Necrotizing fasciitis, caused by “flesh-eating bacteria”, may affect both competent and immunosuppressed patients. It is considered to be a form of cellulitis that rapidly progresses to necrosis of the skin and underlying tissue. It usually occurs after a penetrating injury, followed by development of erythema, blisters, and necrosis. Most of the lesions are caused by Gram-negative bacilli, such as Escherichia coli, Vibrio, Aeromonas, and Gram-positive organisms (Streptococcus, Staphylococcus, Clostridium, Bacteroides). In some cases the infectious organisms may be multiple [9].
Histologically, it ranges from dermal edema with interstitial infiltrate of neutrophils, lymphocyte and macrophages to full destruction of vessels and extensive areas of necrosis. In general the organisms can be seen on H&E although Gram stains may be helpful.
Mortality rate is high and can reach 50%. After diagnosis, it requires immediate treatment with antibiotics and surgical debridement.
Syphilis, as with tuberculosis, has experienced a marked comeback after years of declining incidence [10, 11]. Particularly after the AIDS epidemic, this infection has become much more prevalent to the point that now it is not uncommon to receive skin biopsies with this diagnosis.
Primary and secondary stages of syphilis involve the skin. Primary chancre occurs at the site of inoculation usually 3 weeks after exposure to Treponema pallidum. The chancre usually occurs as a painless, button-like papule that ulcerates centrally. If untreated, systemic dissemination of spirochetes occurs during the secondary stage, up to 6 months after healing of the primary lesion, with a nonpruritic, papulosquamous eruption. In the mucosae the lesions are named condylomata lata, as soft, moist, red to tan, flat-topped papules, nodules, or plaques. A possible diagnostic pitfall is the presence of marked papillomatosis in some of these lesions, mimicking squamous cell carcinoma [12].
Histopathologically, the chancre shows thin to ulcerated epidermis accompanied by a dense dermal infiltrate, usually rich in plasma cells and lymphocytes. The blood vessels typically show endarteritis obliterans (marked endothelial swelling and proliferation associated with infiltration of the vascular wall by inflammatory cells including plasma cells). In secondary syphilis there tends to be a superficial and deep dermal infiltrate with lymphocytes, macrophages, and plasma cells. The epidermis may show acanthosis with spongiosis, psoriasiform hyperplasia, interface dermatitis, or pustules. Previously silver stains were used to detect the spirochetes but now immunohistochemical studies with antibodies against T. pallidum are available making the diagnosis easier [13, 14].
Protozoal infections
Ameba infection still is an unusual disease in the USA, but due to the severity of the infection, which can result in dissemination to the central nervous system and death, it should be considered one of the emergency diagnoses in dermatopathology. It is usually acquired through mucosae. The enteric route may result in perianal lesions. Swimming in infected waters can involve the conjunctiva or sinonasal areas, the latter also a route seen in some patients using nasal rinses with tap water [15]. Clinically, there may be cutaneous painful nodules with subsequent ulceration [16].
Histologically, amebas are difficult to distinguish from macrophages. They are round or oval nucleated organisms (Fig. 5), typically in the areas of necrosis. The CDC has an antibody that helps detect them by immunohistochemistry.
Entities associated with systemic diseases
Pancreatic panniculitis is a type of panniculitis associated with the release of pancreatic lipases into the blood stream. Although rare (2−3% of all patients with pancreatic disorders), it is very important to recognize this pattern since it may be the first sign of pancreatic disease, including pancreatic adenocarcinoma. A common elicited clinical history is that of acute abdominal discomfort/pain one day following consumption of significant amounts of alcohol.
There are tender erythematous plaques and nodules mostly on the lower extremities. Although not frequently mentioned in the Dermatopathology literature, a number of these patients will show also concomitant arthritis [17]. Biopsies show a mostly lobular panniculitis with areas of fat necrosis, saponification and focal calcification [18].
The treatment of pancreatic panniculitis is supportive and depends on the underlying pancreatic pathology.
Erythema nodosum is another panniculitis that may be associated with systemic disease [19]. There are an acute and a chronic form. The acute form shows tender, bright red or dusky red-purple nodules, 1−5 cm, on the anterior surfaces of the lower legs or on the calves, thighs, forearms, hands, and exceptionally the face. The lesions usually do not ulcerate and generally involute within a few weeks. There may be accompanying fever, malaise, leukocytosis, and arthropathy. Acute erythema nodosum occurs in 10−20% of patients with sarcoidosis and is associated with a good prognosis.
The chronic form (erythema nodosum migrans or subacute nodular migratory panniculitis of Vilanova and Piñol) presents one or several red, subcutaneous nodules on the lower leg. Almost all the patients are women with recent history of sore throat and arthralgia. The nodules enlarge by peripheral extension into plaques, often with central clearing. The duration may be from a few months to a few years.
Histologically [20], there is edema of the adipose septa with a lymphohistiocytic infiltrate, with early neutrophils and eosinophils. Frank vasculitis may be seen mostly in cases associated to medications, including estrogenic oral contraceptives. Fat necrosis is not prominent. Miescher radial nodules are clusters of macrophages around small blood vessels, or a slit-like space.
Later lesions show septal panniculitis with widening of the septa inflammation involving the periphery of the fat lobules. There are granulomas with macrophages and multinucleated giant cells. Frank vasculitis and zones of fat necrosis are more common in erythema induratum, a lobular panniculitis associated with tuberculosis although some cases have been reported in patients receiving targeted therapies [21].
Many diseases can be associated with erythema nodosum, including Crohn disease, sarcoidosis, leukemia, and Hodgkin disease. Among the infections it is worth mentioning Streptococcus, Mycobacterium, Yersinia, Brucella, Leptospira, Chlamydia, Coccidioidis, Histoplasma, dermatophytes, Blasto, and toxoplasmosis. Also Herpes simplex, infectious mononucleosis, lymphogranuloma venereum, ornithosis, and psittacosis. Lately, targeted therapies such as Xeloda and newer immunotherapies have also been associated with erythema nodosum [22, 23].
Granulomatosis with polyangiitis (formerly known as Wegener disease) is a systemic disease that comprises a triad of necrotizing granulomatous lesions in the upper (and lower) respiratory tract, necrotizing vasculitis, and focal glomerulonephritis. There may be involvement of conjunctiva and pericardium. Serological studies detect cANCA in many patients [24].
Histologically, there is a mixed infiltrate of lymphocytes, macrophages, and varying numbers of neutrophils and eosinophils. The number of the latter is relatively low, a difference with the prominent eosinophilic infiltrate seen in Churg−Strauss syndrome (disease typically associated with pANCA, and accompanied by asthma, fever, peripheral neuropathy, cardiac lesions, oral ulceration and mild renal disease). The vessels involved are mid-sized. The interstitium contains granulomas without caseating necrosis.
Granulomatous vasculitis associated with inflammatory bowel disease. Up to 10−20% of patients with inflammatory bowel disease patients develop cutaneous manifestations. There may be aphthous stomatitis, finger clubbing, cutaneous polyarteritis nodosa, psoriasis, pyostomatitis vegetans, erythema multiforme and vitiligo. In the skin it usually presents as violaceous papules, nodules and plaques that often ulcerate. Some authors refer to the extraintestinal lesions as “metastatic” Crohn disease.
Histologically, there is usually a superficial and deep infiltrate with lymphocytes, numerous neutrophils, plasma cells, and scattered eosinophils, sometimes diffusely infiltrating the subcutaneous tissue. There may be large areas of confluent necrosis with macrophages and multinucleated giant cells [25]. Some of the cases with more prominent neutrophilic infiltrate resemble Sweet syndrome/pyoderma gangrenosum. Due to the typically dense mononuclear infiltrate, the differential diagnosis includes T- and B-cell lymphomas. Some of the lymphocytes in the subcutaneous tissue may be seen around fat vacuoles, reminiscent of the “rimming” seen in subcutaneous panniculitis-like T-cell lymphoma. A helpful finding is that gene rearrangement studies (IGH and TCR) are negative in this type of vasculitis.
Polyarteritis nodosa is a rare disease of small- and medium-sized muscular arteries in multiple organs, including kidney, heart, liver, gastrointestinal tract, joints and both peripheral and central nervous systems [24]. Cutaneous lesions are seen in 10−15% of cases, typically as palpable purpura and foci of ulceration in the lower limbs.
Histologically, the dermis and subcutis show a perivascular infiltrate with vessel wall damage and a predominance of neutrophils over other inflammatory cells. Extravascular granulomas are exceptional.
Neoplastic lesions
These lesions are important to detect because they may be the first sign of the underlying syndrome [26,27,28].
Cowden or Multiple hamartoma syndrome is associated with abnormalities of the PTEN gene (chromosome 10q). It has multiple trichilemmomas, mostly on the face, and is associated with breast and thyroid neoplasms (thyroid goiter and carcinoma, fibrocystic disease/gynecomastia), leukemia/lymphoma, and a hamartomatous cerebellar lesion, Lhermitte Duclos disease (dysplastic cerebellar gangliocytoma) [29].
Basal cell nevus syndrome (Gorlin syndrome) is an autosomal dominant disease with mutation of the PTCH1 gene on 9q22-31. Patients have early onset of multiple basal cell carcinomas, involving almost all areas of the body. The patients also may develop hamartomatous, follicular-related lesions on the palms and plants (palmoplantar pits), follicular cysts, cutaneous and odontogenic keratocysts, frontal bossing, calcification of the falx cerebri, medulloblastomas, and fibrosarcomas. Very rarely, lesions similar to those of the basal cell nevus syndrome may be limited to an area of the skin, also called linear unilateral basal cell nevus.
Brooke-Spiegler syndrome is associated with multiple cylindromas/spiradenomas. It is autosomal dominant, characterized by a mutation in the CYLD gene on chromosome 16q12-13. The patients often present with multiple tumors, primarily on the head and neck.
Gardner syndrome (familial adenosis polyposis) is characterized by numerous adenomatous polyps in the colon, with a high risk for developing carcinoma. There are mutations in the Adenomatous Polyposis Coli (APC) gene resulting in gastrointestinal polyps (in colon, stomach, and upper small intestine), multiple osteomas, and congenital hypertrophy of retinal pigmented epithelium. Cutaneous findings include follicular cysts (infundibular type, with focal ghost cells as in pilomatricomas or columns of focal parakeratosis as in porokeratosis) [30]. Also frequent are cutaneous and deeply located fibromas and desmoid tumors. A special type is the nuchal-type fibroma, a lesion composed of mature, benign fibrous tissue, intermixed with adipose tissue, and typically presenting on the nape [31].
Muir-Torre syndrome includes development of visceral neoplasms, usually gastrointestinal carcinomas and polyps, but also laryngeal, genitourinary, and hematolymphoid neoplasms [32]. In the skin, these patients have sebaceous neoplasms such as sebaceous adenoma, sebaceoma, and sebaceous carcinoma, some of them with cystic morphology. In addition, there may be multiple cutaneous keratoacanthomas (usually with focal sebaceous differentiation) and standard follicular cysts. Immunohistochemistry may be used to detect loss of expression mismatch repair proteins (MLH1, PMS2, MSH2, and MSH6) in which case the patient is very likely to have the associated syndrome.
Carney complex shows multiple cutaneous myxomas, together with lentiginous lesions and epithelioid blue nevus/epithelioid melanocytoma [33]. These lesions are usually situated in the dermis, with heavily pigmented epithelioid and spindle cells, along with admixed pigment-laden macrophages. The overlying epidermis usually has hyperplasia or ulceration. Despite the relatively high number of positive sentinel lymph nodes in these patients, they seem to mostly behave in a benign fashion. Most of these patients demonstrate loss of expression of the protein kinase A regulatory subunit (R1alpha) (from the PRKAR1A gene on chromosome 17q22-24)
BAP1 loss is a hallmark of a familial cancer susceptibility syndrome (see also the article on melanocytic lesions fron this series). This syndrome is associated with renal cell carcinomas, mesotheliomas, cholangiocarcinomas, cutaneous and uveal melanomas [34]. Since BAP1 expression may be studied by immunohistochemistry, it has been shown that sporadic lesions may happen in patients with no germline mutation [35].
Important epithelial neoplasms
The following lesions may be misdiagnosed as benign due to their low degree of cytologic and architectural atypia.
Digital/Acral papillary adenocarcinoma is a rare and often misdiagnosed malignant tumor of the sweat glands, most commonly encountered on the distal extremities [36]. Although most lesions have a papillary pattern they may be mostly solid with squamous differentiation. In general, they lack significant cytologic atypia and they may only show focal infiltrative pattern at the periphery [37]. Thus, any adnexal tumor of the acral regions should be considered a potential acral papillary adenocarcinoma. Immunohistochemically, these lesions express focal S-100, p63, and CEA. Due to the relatively high metastatic potential of the tumor, it is recommended to pursue wide local excision, sometimes resulting in amputation; some institutions recommend examination of sentinel lymph nodes.
Microcystic adnexal carcinoma is a solitary lesion, on middle-aged individuals, mostly women, on the upper lip or around the mouth. Histologically, it is deeply infiltrative including involvement of the subcutaneous tissue with perineural invasion [38]. The main difficulty in its diagnosis is that the superficial portion shows superficial keratin-filled lumina resembling trichoepithelioma and thus it may be interpreted as a benign lesion. The deeper areas show small, well-formed ducts, resembling syringoma. Perineural invasion is frequent.
Verrucous carcinoma is an epithelial lesion characterized by papillomatosis and low-degree of cytologic atypia [39]. Since they are more commonly seen in sensitive areas (penis, vulva) sometimes the biopsies are superficial, and thus do not allow to observe the characteristic blunt, wide shape of the rete ridges.
Merkel cell carcinoma is a neuroendocrine neoplasm related to the Merkel/Toker cells in the skin. Most lesions occur in elderly individuals associated with solar damage. A second type develops in younger individuals, and is commonly related to infection by a Polyoma virus [40]. The tumor is characterized by basaloid cells and thus can be confused with a basal cell carcinoma, a lesion with much less aggressive behavior than Merkel cell carcinoma. Immunohistochemistry reveals expression of neuroendocrine markers (synaptophysin and chromogranin), CK20, and neurofilaments. A helpful finding is the detection of a dot-like, perinuclear pattern in many of these markers. On the other hand, it is important to remember that up to 15% of basal cell carcinomas may express neuroendocrine markers. In case of doubt, in “basal cell carcinomas” with high numbers of mitotic figures and apoptotic bodies (“starry sky”), we recommend performing a CK20 and, if doubt still persists, a keratin to rule out the dot-like pattern of Merkel cell carcinoma. Analysis of the immune inflammatory infiltrates [41] and of the pattern of metastasis in sentinel lymph nodes [42] improves clinical stratification of patients with cutaneous Merkel cell carcinoma.
Important lymphoproliferative disorders
Blastic plasmacytoid dendritic cell neoplasm (previously known as blastic natural killer cell lymphoma, agranular CD4+ natural killer cell leukemia, agranular CD4+ CD56+ hematodermic neoplasm) is an aggressive neoplasm with differentiation toward a type of plasmacytoid dendritic cell precursors [43]. It may present as papules and nodules on the head and neck, trunk and proximal extremities. Histologically, at low power there are apparently innocuous dermal lesions, with an infiltrate of mononuclear cells showing only relatively moderate degree of cytologic atypia (Fig. 6). Despite their bland appearance, most patients die within one year from diagnosis [44].
The immunohistochemical profile may be deceiving since the cells, despite not having a lymphoid lineage, express CD4 and thus may be confused with mycosis fungoides cells. A mnemonic rule (personal communication; Tetzlaff MT) is “CD123456”, since these tumor cells express CD123, CD4, and CD56 (Fig. 7).
The lesions do not respond to standard antileukemic therapies but there have been some positive responses to targeted therapy against CD123 [45]. Then, a possible diagnostic pitfall is the loss of CD123 expression by the tumor cells in some cases treated with the monoclonal anti-CD123 antibody.
Post-transplant lymphoproliferative disorders usually occur after solid-tumor transplantation but they may be seen in patients receiving other types of immunosuppressive therapies such as methotrexate (treatment of rheumatoid arthritis, psoriasis). These patients develop EBV-related B-cell processes that range all the way from reactive (that often regress after discontinuation of the therapy) to full-blown lymphomas requiring chemotherapy.
The lesions range from macules/papules to nodules with possible ulceration [46]. Histologically, there are mixed infiltrates of lymphocytes, macrophages, plasma cells, neutrophils, and eosinophils. Immunohistochemically, they contain CD3 and CD20 cells [47], sometimes with numerous CD30 cells (likely activated lymphocytes). EBV infection can be detected with immunohistochemistry against EBV-late membrane protein or with in situ hybridization (EBER).
CD8 epidermotropic lymphoproliferative disorders range from low-grade lesions such as lymphomatoid papulosis (type D), mycosis fungoides (usually in young African American and Hispanic females, with good prognosis) to high-grade CD8 cutaneous T-cell lymphomas (Berti lymphoma) [48, 49]. All three can be indistinguishable histologically, based upon the presence of a T-cell (CD3) infiltrate with natural killer features. Thus clinical pathologic correlation is essential (waxing and waning papules in lymphomatoid papulosis; long-standing macules and plaques in CD8 mycosis fungoides [50]; rapidly evolving plaques, with ulceration in CD8 cutaneous lymphoma).
Important melanocytic lesions
It may be stated that all melanocytic lesions are important due to the sometimes difficult distinction between benign and malignant ones, and the relatively aggressive behavior of cutaneous melanoma. However, it is worth highlighting the following entities:
Nevoid melanoma is a term used to describe malignant melanocytic lesions that show many histologic features associated with a diagnosis of nevus: minimal pagetoid upward migration, apparent maturation in the dermis, and small cellular size. However, these lesions tend to show dermal mitotic figures at all levels of the dermis, high proliferation rate with anti-Ki67, and abnormal labeling with HMB45 (patchy pattern in contrast with the zonation pattern with decreased expression from epidermis to dermis seen in nevi) [51, 52].
Regarding the finding of dermal mitotic figures, although they are a very important marker associated with a diagnosis of melanoma, they can also be seen in any melanocytic lesion from very young (less than 1 year of age) individuals and from patients with high hormonal levels (such as pregnant women or receiving hormonal therapy) [53].
Desmoplastic melanoma is sometimes a challenging diagnosis, particularly in those lesions not associated with an obvious intraepidermal component or when dealing with a re-biopsy of a prior lesion since desmoplastic melanoma can mimic a dermal scar. They tend to be amelanotic so the treating physician may not consider this diagnostic possibility in their clinical differential diagnosis.
Histologically, a diagnosis of desmoplastic melanoma should be used for relatively hypocellular lesions composed of spindle cells, with large, hyperchromatic, oval nuclei, within a fibromyxoid stroma [54, 55]. In general, we should be cautious with any specimen, particularly from the head and neck in an elderly individual, with a scar from a prior “biopsy”, which we have not already reviewed. In such cases we have to consider the possibility of using immunohistochemistry with anti-S100 or anti-SOX10, the two markers more likely to be preserved in desmoplastic melanoma. A possible pitfall with these two markers is the detection of positive Schwann cells or scattered, entrapped adnexal glandular cells within the fibrous stroma of a scar. Thus, it is important to determine that most of the spindle cells in the suspicious areas are positive for these markers, and not only a few scattered and isolated cells.
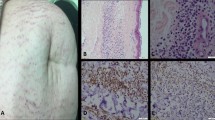
BAP1-deficient lesions (see also above) may be sporadic or associated with a germline mutation of the BAP1 (BRCA1 associated protein 1) gene. These lesions show two distinct components (Fig. 8). A peripheral one has small cells with maturation, consistent with a standard, predominantly intradermal nevus. A second component has large, epithelioid cells, with a resemblance to Spitz nevus cells. They have large, central nuclei, with visible nucleoli. Mitotic figures are very rare in either type of cells [34]. Immunohistochemically, the small, peripheral cells tend to be negative with HMB45 while the central, large cells usually show at least focal HMB45 positivity. There is low proliferation with anti-Ki67 in either component. Both components or only the larger cells will demonstrate loss of BAP1 nuclear expression. Furthermore, in contrast with Spitz nevi, there is expression of BRAF in more than 90% of these lesions. Regarding behavior, most of melanocytic lesions lacking BAP1 expression behave in a benign fashion, although we have seen several cases with subsequent recurrence and metastasis, and therefore qualifying for a diagnosis of melanoma [35].
BAP1-deficient melanocytic nevus. a Intermediate power view of the two components: large, epithelioid cells with a spitzoid morphology and small, standard intradermal nevus cells. b Loss of nuclear expression of BAP1 in the nuclei of lesional cells (expression preserved in the epithelial cells as internal control) (anti-BAP1antibody with light hematoxylin)
Important soft tissue tumors
Epithelioid fibrous histiocytoma is a benign fibrohistiocytic lesion that is important for its possible confusion with melanocytic lesions (see also another article in this series) [56]. It is more common on the trunk and extremities of young adults, between 0.5 and 2 cm in size. Histologically, these lesions are composed of plump, epithelioid cells with abundant eosinophilic cytoplasm and eccentrically placed nuclei with vesicular chromatin and small nucleoli. There is a mostly fibrous stroma, with at least focal thick (keloidal) collagen fibers, mostly at the periphery of the lesion. The overlying epidermis tends to be flat with a peripheral collarette [57].
The lesional cells express the characteristic markers of fibrohistiocytic cells, i.e., CD68, factor XIIIa, CD163, and D2-40, while lacking expression of melanocytic markers (S100, tyrosinase). Interestingly, the tumor cells may express ALK, in relation to the fusion alterations of this gene seen in these lesions [58].
Low-grade fibromyxoid sarcoma is a rare, painless, slow growing tumor commonly situated in the deep soft tissues of the lower extremity, trunk, and upper extremity, more commonly in male adults. This lesion is important because despite its low-grade histology it may present a delayed, but aggressive behavior with distant metastasis. It has a morphologic resemblance with epithelioid fibrosarcoma, although molecular studies seem to suggest that they are separated entities [59, 60].
The lesions appear well circumscribed but may also contain infiltrative areas. There are hypercellular and hypocellular regions with thick-walled, curvilinear vessels. The more cellular areas have a myxoid appearance, with whorls or nodules. The less cellular areas have a more fibrous stroma. The cells are deceptively bland with small hyperchromatic cells, clumped chromatin, and small nucleoli. There are very rare mitotic figures (~1/50 high power fields). The fibrous and myxoid zones can be abruptly separated or else transition between the two. There may be areas with giant rosettes. There are very rare mitotic figures (~1/50 high power fields).
Immunohistochemically, there is strong and diffuse granular labeling for MUC4 [61]. Epithelial membrane antigen, CD99, and BCL2 can be positive, and rarely focal claudin-1 and DOG-1 [62, 63]. These lesions have (7;16)(q34;p11) translocation, resulting in the FUS-CREB3L2 fusion gene and, leass frequently a FUS-CREB3L1 fusion resulting from t(11;16)(p11;p11) or a EWSR1-CREB3L1 fusion [64].
Summary
This chapter describes the diagnostic features of some of the most important lesions in Dermatopathology because they constitute diagnostic emergencies (infections, vasculitis) or are associated with internal malignancies/syndromes (Cowden, Muir-Torre, BAP1, Gardner, Gorlin) or have important differential diagnoses or possible diagnostic pitfalls (acral digital adenocarcinoma, microcystic adnexal carcinoma, Merkel cell carcinoma, blastic plasmodendritic neoplasm, post-transplant lymphoproliferative disorders, CD8 epidermotropic cutaneous lesions, nevoid melanoma, desmoplastic melanoma, epithelioid fibrous histiocytoma, and low-grade fibromyxoid sarcoma). Use of a systematic approach to the diagnosis and consideration of these scenarios in the differential diagnosis of cutaneous biopsies will help establish the correct diagnosis and avoid possible pitfalls.
References
Prieto VG. Cutaneous melanocytic lesions: do not miss the invisible gorilla. Adv Anat Pathol. 2012;19:263–9.
Mays SR, Bogle MA, Bodey GP. Cutaneous fungal infections in the oncology patient: recognition and management. Am J Clin Dermatol. 2006;7:31–43.
Liu K, Howell DN, Perfect JR, Schell WA. Morphologic criteria for the preliminary identification of Fusarium, Paecilomyces, and Acremonium species by histopathology. Am J Clin Pathol. 1998;109:45–54.
Lu XL, Najafzadeh MJ, Dolatabadi S, et al. Taxonomy and epidemiology of Mucor irregularis, agent of chronic cutaneous mucormycosis. Persoonia. 2013;30:48–56.
Bodey GP, Boktour M, Mays S, et al. Skin lesions associated with Fusarium infection. J Am Acad Dermatol. 2002;47:659–66.
Radhakrishnan R, Donato ML, Prieto VG, Mays SR, Raad II, Kuerer HM. Invasive cutaneous fungal infections requiring radical resection in cancer patients undergoing chemotherapy. J Surg Oncol. 2004;88:21–26.
Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609–19.
Sengupta S. Cutaneous herpes zoster. Curr Infect Dis Rep. 2013;15:432–9.
Kim T, Park SY, Kwak YG, et al. Etiology, characteristics, and outcomes of community-onset necrotizing fasciitis in Korea: a multicenter study. PLoS ONE. 2019;14:e0218668.
Ivars Lleo M, Clavo Escribano P, Menendez Prieto B. Atypical cutaneous manifestations in syphilis. Actas Dermosifiliogr. 2016;107:275–83.
Stamm LV. Syphilis: re-emergence of an old foe. Micro Cell. 2016;3:363–70.
Tayal S, Shaban F, Dasgupta K, Tabaqchali MA. A case of syphilitic anal condylomata lata mimicking malignancy. Int J Surg Case Rep. 2015;17:69–71.
Phelps RG, Knispel J, Tu ES, Cernainu G, Saruk M. Immunoperoxidase technique for detecting spirochetes in tissue sections: comparison with other methods. Int J Dermatol. 2000;39:609–13.
Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595–9.
Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018;77:18–22.
Bravo FG, Alvarez PJ, Gotuzzo E. Balamuthia mandrillaris infection of the skin and central nervous system: an emerging disease of concern to many specialties in medicine. Curr Opin Infect Dis. 2011;24:112–7.
Narvaez J, Bianchi MM, Santo P, et al. Pancreatitis, panniculitis, and polyarthritis. Semin Arthritis Rheum. 2010;39:417–23.
Rongioletti F, Caputo V. Pancreatic panniculitis. G Ital Dermatol Venereol. 2013;148:419–25.
Zelger B. Panniculitides, an algorithmic approach. G Ital Dermatol Venereol. 2013;148:351–70.
Thurber S, Kohler S. Histopathologic spectrum of erythema nodosum. J Cutan Pathol. 2006;33:18–26.
Pouldar D, Elsensohn A, Ortenzio F, Shiu J, McLeod M, de Feraudy S. Nodular vasculitis in a patient with Crohn’s disease on vedolizumab. Am J Dermatopathol. 2018;40:e36–e37.
Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy—report of 2 patients. J Cutan Pathol. 2017;44:1080–6.
Martinez-Rodriguez I, Garcia-Castano A, Quirce R, Jimenez-Bonilla J, Banzo I. Erythema nodosum-like panniculitis as a false-positive 18F-FDG PET/CT in advanced melanoma treated with dabrafenib and trametinib. Clin Nucl Med. 2017;42:44–46.
Chasset F, Frances C. Cutaneous manifestations of medium- and large-vessel vasculitis. Clin Rev Allergy Immunol. 2017;53:452–68.
Ko JS, Uberti G, Napekoski K, Patil DT, Billings SD. Cutaneous manifestations in inflammatory bowel disease: a single institutional study of non-neoplastic biopsies over 13 years. J Cutan Pathol. 2016;43:946–55.
Fernandez-Flores A. Skin biopsy in the context of systemic disease. Actas Dermosifiliogr. 2019. https://doi.org/10.1016/j.adengl.2019.07.019.
Schierbeck J, Vestergaard T, Bygum A. Skin cancer associated genodermatoses: a literature review. Acta Derm Venereol. 2019;99:360–9.
Shen Z, Hoffman JD, Hao F, Pier E. More than just skin deep: faciocutaneous clues to genetic syndromes with malignancies. Oncologist . 2012;17:930–6.
Mester J, Charis E. PTEN hamartoma tumor syndrome. Handb Clin Neurol. 2015;132:129–37.
Narisawa Y, Kohda H. Cutaneous cysts of Gardner’s syndrome are similar to follicular stem cells. J Cutan Pathol. 1995;22:115–21.
Michal M, Fetsch JF, Hes O, Miettinen M. Nuchal-type fibroma: a clinicopathologic study of 52 cases. Cancer . 1999;85:156–63.
Gay JT, Gross GP. Muir-Torre Syndrome. In: StatsPearls Publishing LLC,. Treasure Island, FL; 2019. PMID: 30020643.
Leventhal JS, Braverman IM. Skin manifestations of endocrine and neuroendocrine tumors. Semin Oncol. 2016;43:335–40.
Zhang AJ, Rush PS, Tsao H, Duncan LM. BRCA1-associated protein (BAP1) inactivated melanocytic tumors. J Cutan Pathol. 2019. https://doi.org/10.1111/cup.13530. [Epub ahead of print].
Aung PP, Nagarajan P, Tetzlaff MT, et al. Melanoma with loss of BAP1 expression in patients with no family history of BAP1-associated cancer susceptibility syndrome: a case series. Am J Dermatopathol. 2019;41:167–79.
Rismiller K, Knackstedt TJ. Aggressive digital papillary adenocarcinoma: population-based analysis of incidence, demographics, treatment, and outcomes. Dermatol Surg. 2018;44:911–7.
Suchak R, Wang WL, Prieto VG, et al. Cutaneous digital papillary adenocarcinoma: a clinicopathologic study of 31 cases of a rare neoplasm with new observations. Am J Surg Pathol. 2012;36:1883–91.
van der Horst MPJ, Brenn T. Update on malignant sweat gland tumors. Surg Pathol Clin. 2017;10:383–97.
Costache M, Desa LT, Mitrache LE, et al. Cutaneous verrucous carcinoma—report of three cases with review of literature. Rom J Morphol Embryol. 2014;55:383–8.
Kervarrec T, Samimi M, Guyetant S, et al. Histogenesis of Merkel cell carcinoma: a comprehensive review. Front Oncol. 2019;9:451.
Feldmeyer L, Hudgens CW, Ray-Lyons G, et al. Density, distribution, and composition of immune infiltrates correlate with survival in Merkel cell carcinoma. Clin Cancer Res. 2016;22:5553–63.
Ko JS, Prieto VG, Elson PJ, et al. Histological pattern of Merkel cell carcinoma sentinel lymph node metastasis improves stratification of Stage III patients. Mod Pathol. 2016;29:122–30.
Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595–611.
Suzuki Y, Kato S, Kohno K, et al. Clinicopathological analysis of 46 cases with CD4(+) and/or CD56(+) immature haematolymphoid malignancy: reappraisal of blastic plasmacytoid dendritic cell and related neoplasms. Histopathology . 2017;71:972–84.
Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380:1628–37.
Seckin D. Cutaneous lymphoproliferative disorders in organ transplant recipients: update 2014. G Ital Dermatol Venereol. 2014;149:401–8.
Beynet DP, Wee SA, Horwitz SS, et al. Clinical and pathological features of posttransplantation lymphoproliferative disorders presenting with skin involvement in 4 patients. Arch Dermatol. 2004;140:1140–6.
Willemze R, Cerroni L, Kempf W, et al. The2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood. 2019;133:1703–14.
Garcia-Herrera A, Calonje E. Cutaneous lymphomas with cytotoxic phenotype. Surg Pathol Clin. 2017;10:409–27.
Martinez-Escala ME, Kantor RW, Cices A, et al. CD8(+) mycosis fungoides: a low-grade lymphoproliferative disorder. J Am Acad Dermatol. 2017;77:489–96.
Zembowicz A, McCusker M, Chiarelli C, et al. Morphological analysis of nevoid melanoma: a study of 20 cases with a review of the literature. Am J Dermatopathol. 2001;23:167–75.
Scolyer RA, Murali R, McCarthy SW, Thompson JF. Histologically ambiguous (“borderline”) primary cutaneous melanocytic tumors: approaches to patient management including the roles of molecular testing and sentinel lymph node biopsy. Arch Pathol Lab Med. 2010;134:1770–7.
MacKelfresh J, Chen SC, Monthrope YM. Pregnancy and changes in melanocytic nevi. Obstet Gynecol. 2005;106:857–60.
Chen LL, Jaimes N, Barker CA, Busam KJ, Marghoob AA. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825–33.
Broer PN, Walker ME, Goldberg C, et al. Desmoplastic melanoma: a 12-year experience with sentinel lymph node biopsy. Eur J Surg Oncol. 2013;39:681–5.
Glusac EJ, McNiff JM. Epithelioid cell histiocytoma: a simulant of vascular and melanocytic neoplasms. Am J Dermatopathol. 1999;21:1–7.
Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123–9.
Dickson BC, Swanson D, Charames GS, Fletcher CD, Hornick JL. Epithelioid fibrous histiocytoma: molecular characterization of ALK fusion partners in 23 cases. Mod Pathol. 2018;31:753–62.
Patel RM, Downs-Kelly E, Dandekar MN, et al. FUS (16p11) gene rearrangement as detected by fluorescence in-situ hybridization in cutaneous low-grade fibromyxoid sarcoma: a potential diagnostic tool. Am J Dermatopathol. 2011;33:140–3.
Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60–67.
Doyle LA, Wang WL, Dal Cin P, et al. MUC4 is a sensitive and extremely useful marker for sclerosing epithelioid fibrosarcoma: association with FUS gene rearrangement. Am J Surg Pathol. 2012;36:1444–51.
Sambri A, Righi A, Tuzzato G, Donati D, Bianchi G. Low-grade fibromyxoid sarcoma of the extremities: a clinicopathologic study of 24 cases and review of the literature. Pol J Pathol. 2018;69:219–25.
Vallejo-Benitez A, Rodriguez-Zarco E, Carrasco SP, et al. Expression of dog1 in low-grade fibromyxoid sarcoma: a study of 19 cases and review of the literature. Ann Diagn Pathol. 2017;30:8–11.
Mohamed M, Fisher C, Thway K. Low-grade fibromyxoid sarcoma: Clinical, morphologic and genetic features. Ann Diagn Pathol. 2017;28:60–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Although not relevant to the manuscript, V.G.P. has received an honorarium from Myriad MyPath.
Disclosure:
Although not relevant to the manuscript, the author has received an honorarium from Myriad MyPath.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Prieto, V.G. Common traps/pitfalls and emergency diagnosis in dermatopathology. Mod Pathol 33 (Suppl 1), 128–139 (2020). https://doi.org/10.1038/s41379-019-0386-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0386-6