Abstract
Poorly differentiated neoplasms lacking characteristic histopathologic features represent a significant challenge to the pathologist for diagnostic classification. Classically, NUT carcinoma (previously NUT midline carcinoma) is poorly differentiated but typically exhibits variable degrees of squamous differentiation. Diagnosis is genetically defined by NUTM1 rearrangement, usually with BRD4 as the fusion partner. In this multi-institutional next-generation sequencing and fluorescence in situ hybridization study, 26 new NUTM1-rearranged neoplasms are reported, including 20 NUT carcinomas, 4 sarcomas, and 2 tumors of an uncertain lineage. NUTM1 fusion partners were available in 24 of 26 cases. BRD4 was the fusion partner in 18/24 (75%) cases, NSD3 in 2/24 cases (8.3%), and BRD3 in 1/24 (4.2%) cases. Two novel fusion partners were identified: MGA in two sarcomas (myxoid spindle cell sarcoma and undifferentiated sarcoma) (2/24 cases 8.3%) and MXD4 in a round cell sarcoma in the cecum (1/24 cases 4.2%). Eleven cases tested for NUT immunoexpression were all positive, including the MGA and MXD4-rearranged tumors. Our results confirm that NUTM1 gene rearrangements are found outside the classic clinicopathological setting of NUT carcinoma. In addition, as novel fusion partners like MGA and MXD4 may not be susceptible to targeted therapy with bromodomain inhibitors, detecting the NUTM1 rearrangement may not be enough, and identifying the specific fusion partner may become necessary. Studies to elucidate the mechanism of tumorigenesis of novel fusion partners are needed.
Similar content being viewed by others
Introduction
NUT carcinoma (previously known as NUT midline carcinoma) is a rare, aggressive malignancy defined by rearrangement of the NUTM1 (NUT midline carcinoma family member 1) gene on 15q14. Most commonly, NUTM1 is fused with either the bromodomain-containing 4 (BRD4) gene on 19p13.1 or the bromodomain-containing 3 (BRD3) gene on 9q34, and less commonly with the nuclear receptor binding SET domain protein 3 (NSD3) gene. Typical histologic features associated with NUT carcinoma are those of a primitive, epithelial malignancy with variable “abrupt” squamous differentiation. While initially reported in midline structures in young patients, typically in the mediastinum or upper aerodigestive tract, NUT carcinoma is being increasingly recognized in adults and in non-midline anatomic locations such as the kidney, parotid, and thigh [1,2,3]. Prognosis is dismal, with a 6.7-month median overall survival [4]. Traditional chemotherapy and radiation treatments have shown little benefit in treating NUT carcinoma [4]. Bromodomain and extraterminal domain inhibitor therapies have been developed and are currently used in clinical trials.
About 1/3 of NUTM1-rearranged tumors are so-called “NUT variants”, defined as cases harboring fusions of NUTM1 to non-BRD genes, some of which are genes that do not code for or interact with bromodomain-containing proteins. This raises the possibility of some cases of NUTM1-rearranged neoplasia not responding to bromodomain and extraterminal domain inhibitor therapy [4, 5]. Therefore, identification of NUTM1 fusion partners may be essential for appropriate clinical management.
Currently, NUTM1 immunohistochemistry and/or NUTM1 fusion testing is typically limited to cases of poorly differentiated carcinoma with variable squamous differentiation. Recently, however, undifferentiated soft tissue tumors harboring NUTM1 fusions have been identified [2, 3, 6]. Identification of novel histopathologic patterns and fusion partners in NUTM1-rearranged neoplasia not only broadens our understanding of these tumors but also potentially expands the number of patient candidates that may benefit from bromodomain and extraterminal domain inhibitor therapy. Therefore, we set out to describe a multi-institutional experience of NUTM1-rearranged neoplasia.
Materials and methods
Identification of cases
This study was approved by the Institutional Review Boards of all institutions involved. The databases of three institutions (The University of Nebraska Medical Center, Omaha, NE; Caris Life Sciences, Phoenix, AZ; University of Alabama at Birmingham) were searched for cases of NUTM1-rearranged neoplasia. A total of 26 cases were identified.
Thirteen cases (cases 1–13) were from University of Nebraska Medical Center, identified using both NUTM1 break-apart fluorescence in situ hybridization (FISH) analysis, as well as fusion FISH analysis using two-color probe design with probes designed to span the NUTM1 locus and NUTM1 fusion gene loci including MGA, MXD4, WHSC1L1, NSD3, and BRD4.
Cases 14–16 were identified at the University of Alabama at Birmingham, and fusions were detected by next-generation sequencing with the Archer FusionPlex Solid Tumor panel (ArcherDX, Inc., Boulder, CO) at Caris Life Sciences.
Cases 17–25 were identified during routine systematic next-generation sequencing analysis of fusion genes in 14,107 solid malignancies submitted to Caris Life Sciences using the Archer FusionPlex Solid Tumor panel.
Identification of a NUTM1-rearranged case with some histologic overlap with extraskeletal myxoid chondrosarcoma (case 20) prompted one of us (MMM) to screen a previously constructed tissue microarray composed of 31 cases previously diagnosed as extraskeletal myxoid chondrosarcoma for NUT protein expression by immunohistochemistry. This microarray was constructed using previously described [7] methods. This led to the identification of case 26.
FISH studies
FISH studies were performed on 4-µm unstained sections of representative formalin-fixed, paraffin-embedded tissue using a dual-color probe set designed to flank the NUTM1 gene locus (break-apart probe design) and a dual-color probe set designed to span the NUTM1 and NUTM1 fusion gene loci including MGA, MXD4, WHSC1L1, NSD3, and BRD4 (fusion probe design).
Both the custom NUTM1 break-apart probe set and the NUTM1 fusion probe sets utilized cocktails of BAC clones that were selected based on their location in the University of California Santa Cruz Human Genome Browser Gateway (http://genome.ucsc.edu) and were obtained from the BACPAC sources of Children’s Hospital of Oakland Research Institute (Oakland, CA) (http://bacpac.chori.org). Probes were directly labeled by nick translation with either Spectrum Green or Spectrum Orange-dUTP utilizing a modification of the manufacturer’s protocol (Abbott Molecular Inc., Des Plaines, IL) as previously described [8].
Before hybridization, the slides were deparaffinized and then pretreated at room temperature in 0.2 N HCl for 20 min, washed in water for 3 min, incubated at 80 °C for 30 min in VP 2000 Pretreatment Reagent (Abbott Molecular Inc.), and then washed again in H2O for 3 min. Subsequently, the slides were incubated for 30 min at 37 °C in protease solution (25 mg of protease in 50 mL of protease solution) (Abbott Molecular Inc.), washed in 1 × phosphate-buffered saline at room temperature for 5 min, and then dehydrated in gradient ethanol (75%, 85%, and 100%) at room temperature for 1 min each and air-dried. After the cells and probes were codenatured at 80 °C for 5 min and incubated overnight at 37 °C using the ThermoBrite system (Abbott Molecular Inc.), posthybridization washing was performed in 2 × saline-sodium citrate (SSC)/0.3% NP-40 at 72 °C for 2 min, followed by 2 × SSC/0.3% NP-40 at room temperature for 2 min. The slides were then counterstained with 4', 6-diamidino-2-phenylindole (Abbott Molecular Inc.).
Hybridization signals were assessed in 100 interphase nuclei with strong, well-delineated signals for the NUTM1 break-apart and each individual dual fusion probe set, respectively. Only nonoverlapping tumor nuclei with a complete set of signals were scored. As controls, each probe set was also hybridized to metaphase cell preparations of karyotypically normal peripheral blood lymphocytes to confirm correct mapping, optimal signal intensity, and lack of cross-hybridization, before proceeding with analysis of the patient sample. Images were acquired using the Cytovision Image Analysis System (Applied Imaging, Santa Clara, CA). The cutoff level for scoring aberrations was 15% abnormal nuclei for both the dual-color NUTM1 break-apart and dual-color fusion probe systems, based on related in-house validation studies.
Fusion detection
For cases submitted to Caris Life Sciences for gene fusion detection, anchored multiplex PCR was performed for targeted RNA sequencing using the ArcherDx fusion assay (Archer FusionPlex Solid Tumor panel). The formalin-fixed paraffin-embedded tumor samples were microdissected to enrich the sample to ≥ 20% tumor nuclei, and mRNA was isolated and reverse transcribed into complementary DNA (cDNA). Unidirectional gene-specific primers were used to enrich for target regions, followed by next-generation sequencing (Illumina MiSeq platform [Illumina, Sand Diego, CA]). Targets included 52 genes known to be associated with various malignancies. The full list of genes can be found at http://archerdx.com/fusionplex-assays/solid-tumor. Reads and contigs that were matched to a database of known fusions and other oncogenic isoforms (Quiver database, ArcherDx), as well as those novel isoforms or fusions with high reads (> 10% of total reads) and high confidence after bioinformatic filtering, were analyzed. Samples with < 4000 unique RNA reads were reported as indeterminate and excluded from analysis, and all the analyzed fusions were in-frame. Fusions among the > 11,000 fusions known to be found in normal tissues were excluded. The detection sensitivity of the assay allows for detection of a fusion that is present in at least 10% of the cells in the samples tested.
NUT immunohistochemistry
NUT protein expression was evaluated using Leica Bond automation with the AR2 epitope retrieval buffer (25 min). Rabbit monoclonal NUTM1 antibody clone C52B1 (Cell Signaling Technologies Inc., Danvers, MA) was diluted 1:50. Given the multi-institutional nature of this study, only 11 cases had material available for NUT immunohistochemistry. The additional immunohistochemistry data listed in Table 1 were obtained from the outside pathology reports, when available.
Tissue microarray
Immunohistochemistry screening for NUT was performed on the previously constructed tissue microarray composed of 31 cases previously diagnosed as extraskeletal myxoid chondrosarcoma. The slides were both immunostained for NUT and screened for NUTM1 rearrangement using a NUTM1 break-apart FISH probe.
Results
The 26 cases of NUTM1-rearranged tumors are summarized in Table 1. NUTM1 gene rearranged tumors were identified across a wide age range in adolescents and adults (mean age: 44.7 years; range, 12–74; 17 males, 9 females).
NUTM1 fusion genes identified by fusion FISH and the ArcherDx fusion assay
NUTM1 fusion partners were available in 24 of 26 cases. Twenty-one cases (21/24) contained previously described NUTM1 fusion partners. BRD4 was the most common partner, identified in 18 cases (18/24, 75%). Two cases harbored NSD3-NUTM1 fusions (2/24, 8.3%). BRD3-NUTM1 was identified in one case (1/24, 4.2%).
Two of the BRD4-NUTM1 fusion cases (cases 19 and 21) and one NSD3-NUTM1 fusion case (case 23) identified by ArcherDx Fuson Assay were confirmed by fusion FISH analysis. A single patient (case 23) with paired primary (lung) and metastatic (liver) tumor showed an identical NSD3-NUTM1 fusion in both sites.
The fusion partner was not available in two cases (only break-apart FISH results were available on cases 3 and 26).
Novel MGA-NUTM1 and the only recently reported MXD4-NUTM1 were identified by the ArcherDx Fusion Assay (Fig. 1). The MGA-NUTM1 fusion was detected in two cases (2/24, 8.3%; cases 20 and 25) and the MXD4-NUTM1 fusion was detected in one case (1/24, 4.2%; case 22). In cases 20 and 25, the Archer panel detected highly expressed, in-frame MGA-NUTM1 fusions with 162 and 192 unique cDNA start sites, respectively, resulting in exon 21 of MGA (NM_001080541) joined to exon 2 of NUTM1 (NM_175741). MGA and NUTM1 are located 7.4 Mb apart, in opposite orientations on chromosome 15. Therefore, this fusion may have arisen via an inversion. The MXD4-NUTM1 gene fusion in case 22 was in-frame, with exon 5 of MXD4 (NM_006454) joined to exon 2 of NUTM1 (NM_175741). Both fusions preserve the functional domains of MGA and MXD4 and only remove the C-termini of the proteins.
One MGA-NUTM1 fusion case (case 20) was confirmed by fusion FISH analysis.
NUT immunohistochemistry and NUTM1 break-apart FISH on tissue microarray
The extraskeletal myxoid chondrosarcoma tissue microarray identified one case (1/31, 3.2%) with strong nuclear NUT expression by immunohistochemistry. One other case showed focal weak nuclear NUT immunostaining, whereas the rest of the cases in the microarray were completely negative for NUT protein expression.
The case with strong NUT nuclear staining (Table 1, case 26) demonstrated a rearrangement of the NUTM1 locus in 94 of 100 cells evaluated by NUTM1-break-apart FISH, indicating that this case is a NUTM1-rearranged tumor mimicking extraskeletal myxoid chondrosarcoma. In contrast, there was no evidence of a NUTM1 gene rearrangement by FISH in the other cases in the microarray, including the weak NUT expressing case.
Histologic findings
The histomorphology of the NSD3- and most of the BRD4-NUTM1 neoplasms was similar in appearance to what has been previously described in NUT carcinomas. As such, they were composed of sheets of primitive epithelioid cells with a high mitotic rate and variable evidence of squamous differentiation (Fig. 2). However, two poorly differentiated BRD4-NUTM1 fusion tumors (cases 13 and 21) could not be definitively classified as carcinomas, mainly due to insufficient evidence of epithelial differentiation.
The metastatic tumor previously diagnosed as an extraskeletal myxoid chondrosarcoma (case 26) was composed of sheets of round cells (Fig. 3a) and showed nuclear expression of NUT (Fig. 3b) and rearrangement of the NUTM1 gene locus by FISH (Fig. 2, inset). The fusion partner of case 26 could not be determined in the absence of RNA of sufficient quality. Case 26 was classified as a sarcoma based largely on its histologic features.
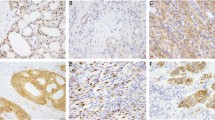
NUTM1-rearranged tumors with variant morphology. a, b Case 26 showed undifferentiated round cells along with NUT expression (NUT IHC in b). This case harbored NUTM1 gene break by break-apart FISH, leading to a diagnosis of NUT-rearranged tumor. c Case 22 showed sheets of epithelioid to plasmacytoid cells with production of dense extracellular collagen type material and harbored an MXD4-NUTM1 fusion. d Case 22 showed nuclear expression of NUT. (a, c, hematoxylin and eosin, ×400 and ×200, respectively; b, d, ×400)
The two cases with novel MGA-NUTM1 fusions were classified as sarcomas. One MGA-fused case (case 20, a lung primary, with liver metastasis) was composed of bland, spindled cells with tapered nuclei in an abundant myxoid matrix (Figs. 4a, b). The other MGA-rearranged case (case 25, chest wall/pleural mass) showed sheets of primitive, round to oval cells with vesicular chromatin and 1–2 prominent nucleoli and scant, nondescript cytoplasm (Figs. 4c, d). The neoplastic cells in case 25 appeared more mesenchymal than epithelial and were associated with foci of hyalinized stroma.
MGA-NUTM1-rearranged cases. a An MGA-NUTM1 case (case 20) showed spindled cells with tapered ends set in an abundant myxoid stroma. b Case 20 also showed speckled pattern of nuclear NUT expression. c, d The other MGA-NUTM1 case (case 25) showed oval to round primitive cells with vesicular nuclei and prominent nucleoli, imparting a more primitive and mesenchymal appearance. (a, c, d, hematoxylin and eosin, ×100, ×200, and ×400, respectively; b, ×200)
The sarcoma with MXD4-NUTM1 fusion (case 22) demonstrated sheets of high-grade epithelioid to plasmacytoid cells with moderate amounts of eosinophilic cytoplasm and focal dense fibrous matrix (Figs. 3c, d), prompting original microscopic interpretation as extraskeletal osteosarcoma. Case 22 was negative for epithelial membrane antigen and pan-cytokeratin, supporting the morphologic impression of sarcoma.
The available immunophenotypic results of these cases are presented in Table 1. Note: given the multi-institutional nature of this study, the immunostains used on the cases in this study were not able to be standardized across all cases. However, a few noteworthy examples are noted here. Six of 14 (6/14; 42.8%) cases showed synaptophysin expression, whereas 0 of 12 cases (0/12) were positive for chromogranin. Of eight cases studied, two (2/8; 25%) expressed TTF1, including both lung and pleural examples.
Immunohistochemistry for NUTM1 protein
Immunohistochemistry results for the NUTM1 protein were available in 11 cases. All 11 cases showed nuclear expression, and often times a dot-like pattern could be discerned within the nuclear staining. This included the two novel MGA-NUTM1 fused cases and the MXD4-NUTM1 fused case.
Discussion
The NUTM1 protein, a protein of largely unknown function, is normally only expressed to any significant degree in the testis, where it shuttles between the nucleus and cytosol. However, when fused to the bromodomain proteins BRD4 or BRD3 in NUT carcinomas, NUTM1 becomes trapped in the nucleus [5, 9]. This is because the bromodomain proteins bind and become localized to acetylated lysine residues on histone proteins. As the NUTM1 protein binds to p300, a histone acetyltransferase, there is sequestration of p300 to sites of BRD4/3-NUTM1 complexes, leading to localized hyperacetylation of histone proteins. This leads to further recruitment of BRD4/3-NUTM1 complexes in a feed-forward mechanism, ultimately forming large contiguous expanses measuring up to two megabases in size that are co-occupied by BRD4/3-NUTM1, p300 and active histone acetylation, so-called “megadomains” [10]. Because acetylation of lysine residues on histone tails is associated with open chromatin and bromodomains are critical in transcription elongation, the fusion protein is preferentially tethered to transcriptionally active DNA resulting in increased transcription of progrowth genes [9]. In contrast, areas away from the megadomains become hypoacetylated, resulting in transcriptional repression of prodifferentiation genes [11, 12]. The BRD4/3-NUTM1 megadomains override the preexisting histone code leading to increased expression of key oncoproteins such as MYC, the stem cell marker SOX2 and TP63 [12,13,14]. The importance of MYC overexpression in NUT carcinoma is evidenced by studies showing that when MYC is force expressed in NUT carcinoma cell lines, differentiation arrest occurs even when BRD4-NUTM1 is knocked down by small interfering RNA [15]. Moreover, bromodomain and extraterminal domain inhibitor therapy represses MYC expression [16]. BRD4-NUTM1 has been shown to drive expression of SOX2, which in turn induces NUT carcinoma cell lines to form stem cell-like spheres with cellular transformation [14]. The increased expression of TP63, a negative regulator of TP53, allows NUT carcinoma cells to evade TP53 [12]. Therefore, NUT carcinomas have a reprogrammed epigenome [4] leading to increased proliferation and arrest of differentiation.
Bromodomain and extraterminal domain inhibitors have been developed that reduce the binding of acetylated histones to BRD4/BRD3, deplete megadomains, and cause cellular differentiation in vitro and in mice [11]. In addition, treatment of NUT carcinoma cell lines with histone deacetylation inhibitors can restore global acetylation, leading to squamous differentiation and arrested growth [11]. At least three clinical trials for bromodomain and extraterminal domain inhibitors are underway as of the writing of this article, and there is a clinical trial for histone deacetylation inhibitors for patients who fail bromodomain and extraterminal domain inhibitor therapy [17]. Indeed, patients with NUT carcinoma have been successfully treated with vorinostat, a Food and Drug Administration-approved histone deacetylation inhibitor [11] and an oral bromodomain and extraterminal domain inhibitor [10]. MYC inhibition therapy with OmoMyc is also being explored in NUT carcinoma [15].
The NSD3 gene, the NUTM1 fusion partner in 10–20% of NUT carcinoma cases [16], encodes a histone methyltransferase protein. Although not a bromodomain protein, NSD3 associates with the extraterminal domain of BRD4 and forms a BRD4-NUTM1-like complex attached to chromatin [10]. In addition, NSD3-NUTM1 containing cells have shown growth arrest and differentiation in response to the bromodomain and extraterminal domain inhibitor JQ1 and therefore the NSD3-NUTM1 fusion protein likely alters epigenetic programming via a similar mechanism as BRD4/3-NUTM1 fusion proteins [16]. Thus, there is rational for bromodomain and extraterminal domain inhibitor treatment in NSD3-NUTM1 cases [10].
In the current study, most NUTM1-rearranged tumors were carcinomas with focal squamous cell differentiation, and BRD4 was the most common fusion partner (18/24, 75%). Many NUT carcinomas in this study were synaptophysin-positive indicating neuroendocrine differentiation, only occasionally reported in NUT carcinomas [18]. Two NUT carcinomas with NSD3 fusions and one case with a BRD3 fusion were also identified. Moreover, an MXD4-NUTM1 and novel MGA-NUTM1 fusions were identified in one and two cases, respectively. Interestingly, MAX dimerization protein 4 (MXD4) and MAX gene-associated protein (MGA) are both members of the MAX-interacting transcription factor network, which includes proteins that engage MAX as a cofactor for DNA binding and control of gene expression. Heterodimerization of MXD4 or MGA with MAX result in transcriptional repression of E-box target DNA sequences [19]. Immunohistochemical staining of our MXD4-NUTM1 and MGA-NUTM1 fusion cases with NUTM1 antibody showed distinct nuclear foci of NUTM1 protein accumulation, not unlike those seen in BRD4-NUTM1 fusion cases [20], suggesting that NUTM1-rearranged tumors with MXD4 and MGA fusion partners may share similar oncogenic mechanisms as BRD3/BRD4-NUTM1 cases. In contrast, no NUTM1 immunoexpression was observed in a single MXD1-NUTM1 fusion positive NUT carcinoma reported by Dickson et al. utilizing the same antibody as used in our study. These authors, however, did demonstrate high levels of mRNA expression of NUTM1 and accordingly hypothesized that a posttranscriptional mechanism may be responsible for the lack of NUTM1 immunostaining [2].
Given that only the C-termini of both MXD4 and MGA are lost as a result of the fusion resulting in the functional domains of both proteins (T-Box in MGA and bHLH in MGA and MXD4) remaining intact (Fig. 1), a possible mechanism of bringing MXD4-NUTM1 and MGA-NUTM1 fusion proteins to specific areas of the nucleus would be through heterodimerization with MAX and binding of the heterodimers to the DNA. This would tether NUTM1 to specific areas of the nucleus where it could attract p300 resulting in acetylation of neighboring histones. This would imply a bromodomain and extraterminal motif family-independent mechanism of NUTM1 recruitment to the nucleus, which has thus far not been described in the literature. Studies of novel NUTM1 fusion proteins such as ZNF532-NUTM1 and NSD3-NUTM1 have found interactions between these proteins and BRD4 [20, 21] but to the best of our knowledge, no interactions have been described between the MAX-interacting transcription factor network and BRD4 complexes or between the MAX protein and BRD4 complexes. Additional studies are needed to elucidate if MXD4-NUTM1, MGA-NUTM1 and the recently reported MXD1-NUTM1 and BCORL1-NUTM1 fusion proteins [2] act through a bromodomain and extraterminal motif family-independent mechanism. If so, this would have treatment implications as bromodomain and extraterminal domain inhibitors may not have the same effect on malignancies harboring these novel, bromodomain and extraterminal motif-independent fusions [22].
There were four NUTM1-rearranged tumors that were classified as sarcomas. Three of these tumors featured either MGA-NUTM1 (two cases) or MXD4-NUTM1 (one case) fusions. One of the MGA-NUTM1 cases (case 20) showed spindled cells in a myxoid stroma, with resemblance to extraskeletal myxoid chondrosarcoma. The other MGA-NUTM1 case (case 25) and the MXD4-NUTM1-rearranged cecal tumor showed small round cell features, and were keratin negative. Recently, Tamura et al. reported an MXD4-NUTM1 fusion in a fatal ovarian sarcoma that featured small round cells with abundant eosinophilic cytoplasm, not unlike our MXD4-NUTM1-rearranged sarcoma that arose in the cecum (case 22) [23]. Our MXD4-NUTM1 cecal tumor also shared histologic features with the gastric tumor that harbored an MXD1-NUTM1 fusion as reported in Dickson et al. [2].
In addition, neoplasms with sarcomatous features have been reported in association with NUTM1 fusions with traditional partners such as BRD4. Den Bakker et al. [3] reported a BRD4-NUTM1-rearranged tumor in the parotid gland that showed carcinoma, which transitioned into mesenchymal and chondroid areas, the latter of which were negative for keratins and p63. Dickson et al. [2] recently reported on soft tissue tumors harboring NUTM1 rearrangements, at least four of which could potentially be considered sarcomas. Patient 4 of that study harbored a BRD4-NUTM1 fusion but was negative for multiple epithelial markers and histologically showed spindled cells organized in a reticular pattern set in a myxoid stroma, not unlike our case 20. Patient 5 also harbored a BRD4-NUTM1 fusion, was located in the kidney, showed only focal weak expression of keratins and histologically featured sheets of round to rhabdoid cells. Patient 2 was intramuscular, contained a BCORL1-NUTM1 fusion, contained areas with chondromyxoid stroma not too different from our case 20 (see Fig. 2d of Dickson et al. [2]) and was negative for multiple keratins. Finally, Patient 3 had a gastric tumor that carried a MXD1-NUTM1 fusion and histologically showed sheets of eosinophilic rhabdoid cells, which judging by Figs. 3a-c of Dickson et al. is not too dissimilar from our case 22, which arose in the cecal area and contained an MXD4-NUTM1 fusion. Additional experience with NUTM1-rearranged neoplasia may shed light on a possible association between NUTM1-rearranged gastrointestinal tumors and MXD genes as fusion partners. Furthermore, recent descriptions of highly aggressive CIC-NUTM1 sarcomas have been described involving the central nervous system, bone, soft tissue and viscera, usually in younger patients [24, 25]. Microscopically they feature monomorphic cells, mostly round, but spindled, epithelioid, and plasmacytoid/rhabdoid cytology can be seen. Tumor cells may grow in sheets with vague lobularity, nests, trabecular, and/or reticular architectures. Stroma is typically scanty in CIC-NUTM1 sarcomas but can be myxoid. Thus, CIC-NUTM1 sarcomas show histologic overlap with CIC-DUX4 sarcoma, NUT carcinomas, and malignant myoepithelial tumors. Interestingly, however, using transcriptomics data, CIC-NUTM1 sarcomas are distinct from NUT carcinomas but are indistinguishable from other CIC-fused sarcomas. Like NUT carcinomas, CIC-NUTM1 sarcomas have shown consistent staining for NUT using the C52B1 clone, whereas CIC-DUX4 sarcomas, BCOR-rearranged sarcomas, and Ewing sarcomas are negative for this antibody [24].
The identification of a MGA-NUTM1 fusion-positive neoplasm in the current study that morphologically resembled extraskeletal myxoid chondrosarcoma (case 20) prompted the staining of a previously constructed tissue microarray composed of 31 cases previously diagnosed as extraskeletal myxoid chondrosarcoma for the NUT immunostain. Notably, 1 of the 31 cases exhibited strong nuclear NUT immunoexpression and was subsequently confirmed to have a rearrangement of the NUTM1 locus by FISH. These findings, as well as those of Dickson et al. [2] support an expanded histologic spectrum of NUTM1-rearranged neoplasia.
Case 11 is the first NUT carcinoma, to our knowledge, to involve the ovary. However, given widespread disease in this patient, it was not clear if the lung or ovary was the primary site in this case. This case and cases 3, 20, 22, 25, and 26 reinforce the concept that NUTM1-rearranged neoplasia may be located outside of the anatomic midline.
In summary, most NUTM1-rearranged tumors are carcinomas containing BRD4-NUTM1 or NSD3-NUTM1 fusions, and they often feature focal squamous cell and sometimes neuroendocrine differentiation. The novel NUTM1 fusion partner MGA and the only recently reported MXD4 fusion partner were identified in tumors showing sarcomatous morphology. Prior to molecular assessment, only 4 of the 26 cases were initially suspected to be NUTM1-rearranged tumors emphasizing the value of advanced molecular techniques in solidifying diagnostic classification and providing treatment opportunities such as bromodomain and extraterminal domain inhibitor therapy or related appropriate clinical trial. NUTM1 immunostaining for suspect cases, including those outside the classic presentation of a midline poorly differentiated carcinoma with “abrupt” squamous differentiation should be considered [18]. If positive, molecular confirmation to include identification of the specific fusion partner by FISH and/or next-generation sequencing is necessary, since it is currently not known if the novel fusion partners respond to bromodomain and extraterminal motif inhibition.
References
Sirohi D, Garg K, Simko JP, Grenert JP. Renal NUT carcinoma: a case report. Histopathology. 2018;72:528–30.
Dickson BC, Sung YS, Rosenblum MK, Reuter VE, Harb M, Wunder JS, et al. NUTM1 gene fusions characterize a subset of undifferentiated soft tissue and visceral tumors. Am J Surg Pathol. 2018;42:636–45.
den Bakker MA, Beverloo BH, van den Heuvel-Eibrink MM, Meeuwis CA, Tan LM, Johnson LA, et al. NUT midline carcinoma of the parotid gland with mesenchymal differentiation. Am J Surg Pathol. 2009;33:1253–8.
Bauer DE, Mitchell CM, Strait KM, Lathan CS, Stelow EB, Lüer SC, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–9.
French CA. Demystified molecular pathology of NUT midline carcinomas. J Clin Pathol. 2010;63:492–6.
Mertens F, Wiebe T, Adlercreutz C, Mandahl N, French CA. Successful treatment of a child with t(15;19)-positive tumor. Pediatr Blood Cancer. 2007;49:1015–7.
Miettinen M. A simple method for generating multitissue blocks without special equipment. Appl Immunohistochem Mol Morphol. 2012;20:410–2.
Huang D, Sumegi J, Dal Cin P, Reith JD, Yasuda T, Nelson M, et al. C11orf95-MKL2 is the resulting fusion oncogene of t(11;16) (q13;p13) in chondroid lipoma. Genes Chromosomes Cancer. 2010;49:810–8.
French CA, Ramirez CL, Kolmakova J, Hickman TT, Cameron MJ, Thyne ME, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–42.
Stathis A, Zucca E, Bekradda M, Gomez-Roca C, Delord JP, de La Motte Rouge T, et al. Clinical response of carcinomas harboring the BRD4-NUT oncoprotein to the targeted bromodomain Inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500.
Schwartz BE, Hofer MD, Lemieux ME, Bauer DE, Cameron MJ, West NH, et al. Differentiation of NUT midline carcinoma by epigenomic reprogramming. Cancer Res. 2011;71:2686–96.
Alekseyenko AA, Walsh EM, Wang X, Grayson AR, Hsi PT, Kharchenko PV, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29:1507–23.
Zee BM, Dibona AB, Alekseyenko AA, French CA, Kuroda MI. The oncoprotein BRD4-NUT generates aberrant histone modification patterns. PLoS ONE. 2016;11:e0163820.
Wang R, Liu W, Helfer CM, Bradner JE, Hornick JL, Janicki SM, et al. Activation of SOX2 expression by BRD4-NUT oncogenic fusion drives neoplastic transformation in NUT midline carcinoma. Cancer Res. 2014;74:3332–43.
Grayson AR, Walsh EM, Cameron MJ, Godec J, Ashworth T, Ambrose JM, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33:1736–42.
French CA, Rahman S, Walsh EM, Kühnle S, Grayson AR, Lemieux ME, et al. NSD3-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014;4:928–41.
Chau NG, Hurwitz S, Mitchell CM, Aserlind A, Grunfeld N, Kaplan L, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016;122:3632–40.
Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–21.
Cascon A, Robledo M. MAX and MYC: a heritable breakup. Cancer Res. 2012;72:3119–24.
Alekseyenko AA, Walsh EM, Zee BM, Pakozdi T, Hsi P, Lemieux ME, et al. Ectopic protein interactions within BRD4-chromatin complexes drive oncogenic megadomain formation in NUT midline carcinoma. Proc Natl Acad Sci USA. 2017;114:E4184–E92.
Rahman S, Sowa ME, Ottinger M, Smith JA, Shi Y, Harper JW, et al. The Brd4 extraterminal domain confers transcription activation independent of pTEFb by recruiting multiple proteins, including NSD3. Mol Cell Biol. 2011;31:2641–52.
Stathis A, Bertoni F. BET proteins as targets for anticancer treatment. Cancer Discov. 2018;8:24–36.
Tamura R, Nakaoka H, Yoshihara K, Mori Y, Yachida N, Nishikawa N, et al. Novel MXD4–NUTM1 fusion transcript identified in primary ovarian undifferentiated small round cell sarcoma. Genes Chromosomes Cancer. 2018;57:557–63.
Le Loarer F, Pissaloux D, Watson S, Godfraind C, Galmiche-Rolland L, Silva K, et al. Clinicopathologic features of CIC-NUTM1 sarcomas, a new molecular variant of the family of CIC-fused sarcomas. Am J Surg Pathol. 2019;43:268–76.
Schaefer IM, Dal Cin P, Landry LM, Fletcher CDM, Hanna GJ, French CA. CIC‐NUTM1 fusion: a case which expands the spectrum of NUT‐rearranged epithelioid malignancies. Genes Chromosomes Cancer. 2018;57:446–51.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stevens, T.M., Morlote, D., Xiu, J. et al. NUTM1-rearranged neoplasia: a multi-institution experience yields novel fusion partners and expands the histologic spectrum. Mod Pathol 32, 764–773 (2019). https://doi.org/10.1038/s41379-019-0206-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41379-019-0206-z
This article is cited by
-
Unusual lung tumors—from morphology to genetics
Modern Pathology (2022)
-
The role of NSD1, NSD2, and NSD3 histone methyltransferases in solid tumors
Cellular and Molecular Life Sciences (2022)
-
Adamantinoma-Like Ewing Sarcoma of the Head and Neck: A Case-Series of a Rare and Challenging Diagnosis
Head and Neck Pathology (2022)
-
Feasibility of whole genome and transcriptome profiling in pediatric and young adult cancers
Nature Communications (2022)
-
Incidence of NUT carcinoma in Western Australia from 1989 to 2014: a review of pediatric and adolescent cases from Perth Children’s Hospital
BMC Cancer (2021)