Abstract
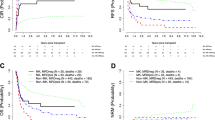
Baseline cytogenetic studies at diagnosis remain the single most important determinant of outcome in patients with acute myeloid leukemia (AML). However, the prognostic role of the complete gamut of cytogenetic aberrations in AML patients undergoing allogeneic hematopoietic stem cell transplantation (allo-HSCT) is currently undefined. In addition, their significance in conjunction with FLT3-ITD status has not been addressed thus far. Using the ALWP/EBMT registry we conducted a retrospective analysis to determine the clinical outcomes of AML patients undergoing allo-HSCT with respect to specific recurring cytogenetic abnormalities complemented with FLT3-ITD status. We analyzed a cohort consisting of 8558 adult AML patients who underwent allo-HSCT from either a matched sibling or a matched unrelated donor. Patients with inv(3)(q21q26)/t(3;3)(q21;q26), del(5q), monosomy 7, chromosome 17p abnormalities, t(10;11)(p11-14;q13-23), t(6;11)(q27;q23), as well as those patients with a monosomal or complex karyotype experienced significantly inferior leukemia-free survival (LFS) compared to patients with a normal karyotype. Trisomy 14, del(9q), and loss of chromosome X were associated with improved LFS rates. A novel prognostic model delineating 5 distinct groups incorporating cytogenetic complexity and FLT3-ITD status was constructed with significant prognostic implications. Our analysis supports the added prognostic significance of FLT3-ITD to baseline cytogenetics in patients undergoing allo-HSCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, Welch JS, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–10.
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, et al. The origin and evolution of mutations in acute myeloid leukemia. Cell. 2012;150: 264–78.
Cancer Genome Atlas Research N, Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74.
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature. 2014;506:328–33.
Lindsley RC, Mar BG, Mazzola E, Grauman PV, Shareef S, Allen SL, et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood. 2015;125:1367–76.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med. 2016;374:2209–21.
Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016;127:62–70.
Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–47.
Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000;96:4075–83.
Ciurea SO, Labopin M, Socie G, Volin L, Passweg J, Chevallier P, et al. Relapse and survival after transplantation for complex karyotype acute myeloid leukemia: A report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation and the University of Texas MD Anderson Cancer Center. Cancer. 2018;124; 2134–41.
Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH, et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. J Clin Oncol. 2008;26:4791–7.
Cornelissen JJ, Breems D, van Putten WL, Gratwohl AA, Passweg JR, Pabst T, et al. Comparative analysis of the value of allogeneic hematopoietic stem-cell transplantation in acute myeloid leukemia with monosomal karyotype versus other cytogenetic risk categories. J Clin Oncol. 2012;30:2140–6.
Brands-Nijenhuis AV, Labopin M, Schouten HC, Volin L, Socie G, Cornelissen JJ, et al. Monosomal karyotype as an adverse prognostic factor in patients with acute myeloid leukemia treated with allogeneic hematopoietic stem-cell transplantation in first complete remission: a retrospective survey on behalf of the ALWP of the EBMT. Haematologica. 2016;101:248–55.
Kurosawa S, Miyawaki S, Yamaguchi T, Kanamori H, Sakura T, Moriuchi Y, et al. Prognosis of patients with core binding factor acute myeloid leukemia after first relapse. Haematologica. 2013;98:1525–31.
Hospital MA, Prebet T, Bertoli S, Thomas X, Tavernier E, Braun T, et al. Core-binding factor acute myeloid leukemia in first relapse: a retrospective study from the French AML Intergroup. Blood. 2014;124:1312–9.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116:354–65.
Stolzel F, Mohr B, Kramer M, Oelschlagel U, Bochtler T, Berdel WE, et al. Karyotype complexity and prognosis in acute myeloid leukemia. Blood Cancer J. 2016;6:e386.
Kang L, Chen W, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34:685–703.
Li S, Garrett-Bakelman FE, Chung SS, Sanders MA, Hricik T, Rapaport F, et al. Distinct evolution and dynamics of epigenetic and genetic heterogeneity in acute myeloid leukemia. Nat Med. 2016;22:792–9.
Wang M, Lindberg J, Klevebring D, Nilsson C, Mer AS, Rantalainen M, et al. Validation of risk stratification models in acute myeloid leukemia using sequencing-based molecular profiling. Leukemia. 2017;31:2029–36.
Herold T, Jurinovic V, Batcha AMN, Bamopoulos SA, Rothenberg-Thurley M, Ksienzyk B, et al. A 29-gene and cytogenetic score for the prediction of resistance to induction treatment in acute myeloid leukemia. Haematologica. 2018;103:456–65.
Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100:4325–36.
Hackl H, Astanina K, Wieser R. Molecular and genetic alterations associated with therapy resistance and relapse of acute myeloid leukemia. J Hematol Oncol. 2017;10:51.
Brissot E, Labopin M, Stelljes M, Ehninger G, Schwerdtfeger R, Finke J, et al. Comparison of matched sibling donors versus unrelated donors in allogeneic stem cell transplantation for primary refractory acute myeloid leukemia: a study on behalf of the Acute Leukemia Working Party of the EBMT. J Hematol Oncol. 2017;10:130.
Krauter J, Wagner K, Schafer I, Marschalek R, Meyer C, Heil G, et al. Prognostic factors in adult patients up to 60 years old with acute myeloid leukemia and translocations of chromosome band 11q23: individual patient data-based meta-analysis of the German Acute Myeloid Leukemia Intergroup. J Clin Oncol. 2009;27:3000–6.
Bhatnagar B, Blachly JS, Kohlschmidt J, Eisfeld AK, Volinia S, Nicolet D, et al. Clinical features and gene- and microRNA-expression patterns in adult acute leukemia patients with t(11;19)(q23; p13.1) and t(11;19)(q23;p13.3). Leukemia. 2016;30:1586–9.
Chen Y, Kantarjian H, Pierce S, Faderl S, O’Brien S, Qiao W, et al. Prognostic significance of 11q23 aberrations in adult acute myeloid leukemia and the role of allogeneic stem cell transplantation. Leukemia. 2013;27:836–42.
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Dumezy F, Renneville A, Mayeur-Rousse C, Nibourel O, Labis E, Preudhomme C. Acute myeloid leukemia with translocation t(3;5): new molecular insights. Haematologica. 2013;98:e52–4.
Johansson B, Billstrom R, Mauritzson N, Mitelman F. Trisomy 19 as the sole chromosomal anomaly in hematologic neoplasms. Cancer Genet Cytogenet. 1994;74:62–5.
Peniket AJ. Del(9q) acute myeloid leukaemia: clinical and cytological characteristics and prognostic implications. Br J Haematol. 2005;130:969. author reply
Prebet T, Boissel N, Reutenauer S, Thomas X, Delaunay J, Cahn JY, et al. Acute myeloid leukemia with translocation (8;21) or inversion (16) in elderly patients treated with conventional chemotherapy: a collaborative study of the French CBF-AML intergroup. J Clin Oncol. 2009;27:4747–53.
Kuchenbauer F, Schnittger S, Look T, Gilliland G, Tenen D, Haferlach T, et al. Identification of additional cytogenetic and molecular genetic abnormalities in acute myeloid leukaemia with t(8;21)/AML1-ETO. Br J Haematol. 2006;134:616–9.
Cui W, Bueso-Ramos CE, Yin CC, Sun J, Chen S, Muddasani R, et al. Trisomy 14 as a sole chromosome abnormality is associated with older age, a heterogenous group of myeloid neoplasms with dysplasia, and a wide spectrum of disease progression. J Biomed Biotechnol. 2010;2010:365318.
Kuwatsuka Y, Miyamura K, Suzuki R, Kasai M, Maruta A, Ogawa H, et al. Hematopoietic stem cell transplantation for core binding factor acute myeloid leukemia: t(8;21) and inv(16) represent different clinical outcomes. Blood. 2009;113:2096–103.
The 44th Annual Meeting of the European Society for Blood and Marrow Transplantation: Physicians Oral Session. Bone Marrow Transplantation. 2018; https://doi.org/10.1038/s41409-018-0318-y.
Ishiyama K, Takami A, Kanda Y, Nakao S, Hidaka M, Maeda T, et al. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9)(p23; q34) dramatically improves the patient prognosis: a matched-pair analysis. Leukemia. 2012;26:461–4.
Schlenk RF, Dohner K, Krauter J, Frohling S, Corbacioglu A, Bullinger L, et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N Engl J Med. 2008;358:1909–18.
Brunet S, Labopin M, Esteve J, Cornelissen J, Socie G, Iori AP, et al. Impact of FLT3 internal tandem duplication on the outcome of related and unrelated hematopoietic transplantation for adult acute myeloid leukemia in first remission: a retrospective analysis. J Clin Oncol. 2012;30:735–41.
Schmid C, Labopin M, Socie G, Daguindau E, Volin L, Huynh A, et al. Outcome of patients with distinct molecular genotypes and cytogenetically normal AML after allogeneic transplantation. Blood. 2015;126:2062–9.
Haferlach C, Alpermann T, Schnittger S, Kern W, Chromik J, Schmid C, et al. Prognostic value of monosomal karyotype in comparison to complex aberrant karyotype in acute myeloid leukemia: a study on 824 cases with aberrant karyotype. Blood. 2012;119:2122–5.
Pasquini MC, Zhang MJ, Medeiros BC, Armand P, Hu ZH, Nishihori T, et al. Hematopoietic cell transplantation outcomes in monosomal karyotype myeloid malignancies. Biol Blood Marrow Transplant. 2016;22:248–57.
Yanada M, Kurosawa S, Yamaguchi T, Yamashita T, Moriuchi Y, Ago H, et al. Prognosis of acute myeloid leukemia harboring monosomal karyotype in patients treated with or without allogeneic hematopoietic cell transplantation after achieving complete remission. Haematologica. 2012;97:915–8.
Poire X, Labopin M, Cornelissen JJ, Volin L, Richard Espiga C, Veelken JH, et al. Outcome of conditioning intensity in acute myeloid leukemia with monosomal karyotype in patients over 45 year-old: A study from the acute leukemia working party (ALWP) of the European group of blood and marrow transplantation (EBMT). Am J Hematol. 2015;90:719–24.
Kayser S, Zucknick M, Dohner K, Krauter J, Kohne CH, Horst HA, et al. Monosomal karyotype in adult acute myeloid leukemia: prognostic impact and outcome after different treatment strategies. Blood. 2012;119:551–8.
Fang M, Storer B, Estey E, Othus M, Zhang L, Sandmaier BM, et al. Outcome of patients with acute myeloid leukemia with monosomal karyotype who undergo hematopoietic cell transplantation. Blood. 2011;118):1490–4.
Strickland SA, Sun Z, Ketterling RP, Cherry AM, Cripe LD, Dewald G, et al. Independent Prognostic Significance Of Monosomy 17 and Impact of Karyotype Complexity in Monosomal Karyotype/Complex Karyotype Acute Myeloid Leukemia: Results from Four ECOG-ACRIN Prospective Therapeutic Trials. Leuk Res. 2017;59:55–64.
Armand P, Kim HT, Zhang MJ, Perez WS, Dal Cin PS, Klumpp TR, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18:280–8.
Medeiros BC, Othus M, Fang M, Appelbaum FR, Erba HP. Cytogenetic heterogeneity negatively impacts outcomes in patients with acute myeloid leukemia. Haematologica. 2015;100:331–5.
Acknowledgements
We thank all the European Group for Blood and Marrow Transplantation (EBMT) centers and national registries for contributing patients to the study and data managers for their excellent work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Canaani, J., Labopin, M., Itälä-Remes, M. et al. Prognostic significance of recurring chromosomal abnormalities in transplanted patients with acute myeloid leukemia. Leukemia 33, 1944–1952 (2019). https://doi.org/10.1038/s41375-019-0439-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0439-3
This article is cited by
-
Matched related versus unrelated versus haploidentical donors for allogeneic transplantation in AML patients achieving first complete remission after two induction courses: a study from the ALWP/EBMT
Bone Marrow Transplantation (2023)
-
Non-T depleted haploidentical stem cell transplantation in AML patients achieving first complete remission after one versus two induction courses: a study from the ALWP/EBMT
Bone Marrow Transplantation (2022)
-
Cytogenetics analysis as the central point of genetic testing in acute myeloid leukemia (AML): a laboratory perspective for clinical applications
Clinical and Experimental Medicine (2022)
-
Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022
Bone Marrow Transplantation (2022)
-
Outcome of human umbilical cord blood stem cell transplantation (CBT) for acute myeloid leukemia in patients achieving first complete remission after one versus two induction courses: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2022)