Abstract
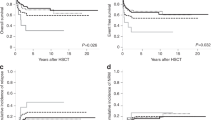
Poor-risk (PR) cytogenetic/molecular abnormalities generally direct pediatric patients with acute myeloid leukemia (AML) to allogeneic hematopoietic stem cell transplant (HSCT). We assessed the predictive value of cytogenetic risk classification at diagnosis with respect to post-HSCT outcomes in pediatric patients. Patients younger than 18 years at the time of their first allogeneic HSCT for AML in CR1 between 2005 and 2022 who were reported to the European Society for Blood and Marrow Transplantation registry were subgrouped into four categories. Of the 845 pediatric patients included in this study, 36% had an 11q23 abnormality, 24% had monosomy 7/del7q or monosomy 5/del5q, 24% had a complex or monosomal karyotype, and 16% had other PR cytogenetic abnormalities. In a multivariable model, 11q23 (hazard ratio [HR] = 0.66, P = 0.03) and other PR cytogenetic abnormalities (HR = 0.55, P = 0.02) were associated with significantly better overall survival when compared with monosomy 7/del7q or monosomy 5/del5q. Patients with other PR cytogenetic abnormalities had a lower risk of disease relapse after HSCT (HR = 0.49, P = 0.01) and, hence, better leukemia-free survival (HR = 0.55, P = 0.01). Therefore, we conclude that PR cytogenetic abnormalities at diagnosis predict overall survival after HSCT for AML in pediatric patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
Deidentified dataset maybe shared upon request to the corresponding author.
References
Rubnitz JE, Kaspers GJL. How I treat pediatric acute myeloid leukemia. Blood. 2021;138:1009–18.
Loken MR, Alonzo TA, Pardo L, Gerbing RB, Raimondi SC, Hirsch BA, et al. Residual disease detected by multidimensional flow cytometry signifies high relapse risk in patients with de novo acute myeloid leukemia: a report from Children’s Oncology Group. Blood. 2012;120:1581–8.
Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29:551–65.
Rubnitz JE. Current management of childhood acute myeloid leukemia. Paediatr Drugs. 2017;19:1–10.
Balgobind BV, Raimondi SC, Harbott J, Zimmermann M, Alonzo TA, Auvrignon A, et al. Novel prognostic subgroups in childhood 11q23/MLL-rearranged acute myeloid leukemia: results of an international retrospective study. Blood. 2009;114:2489–96.
Horan JT, Alonzo TA, Lyman GH, Gerbing RB, Lange BJ, Ravindranath Y, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: the Children’s Oncology Group. J Clin Oncol. 2008;26:5797–801.
Pollard JA, Guest E, Alonzo TA, Gerbing RB, Loken MR, Brodersen LE, et al. Gemtuzumab Ozogamicin improves event-free survival and reduces relapse in pediatric KMT2A-rearranged AML: results from the Phase III children’s oncology group trial AAML0531. J Clin Oncol. 2021;39:3149–60.
van Weelderen RE, Klein K, Harrison CJ, Jiang Y, Abrahamsson J, Arad-Cohen N, et al. Measurable residual disease and fusion partner independently predict survival and relapse risk in childhood KMT2A-rearranged acute myeloid leukemia: a study by the international Berlin-Frankfurt-Munster Study Group. J Clin Oncol. 2023;41:2963–74.
Coenen EA, Zwaan CM, Reinhardt D, Harrison CJ, Haas OA, de Haas V, et al. Pediatric acute myeloid leukemia with t(8;16)(p11;p13), a distinct clinical and biological entity: a collaborative study by the International-Berlin-Frankfurt-Munster AML-study group. Blood. 2013;122:2704–13.
Harrison CJ, Hills RK, Moorman AV, Grimwade DJ, Hann I, Webb DK, et al. Cytogenetics of childhood acute myeloid leukemia: United Kingdom Medical Research Council Treatment trials AML 10 and 12. J Clin Oncol. 2010;28:2674–81.
Marceau-Renaut A, Duployez N, Ducourneau B, Labopin M, Petit A, Rousseau A, et al. Molecular profiling defines distinct prognostic subgroups in childhood AML: a report from the french ELAM02 Study Group. Hemasphere. 2018;2:e31.
Masetti R, Pigazzi M, Togni M, Astolfi A, Indio V, Manara E, et al. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood. 2013;121:3469–72.
von Neuhoff C, Reinhardt D, Sander A, Zimmermann M, Bradtke J, Betts DR, et al. Prognostic impact of specific chromosomal aberrations in a large group of pediatric patients with acute myeloid leukemia treated uniformly according to trial AML-BFM 98. J Clin Oncol. 2010;28:2682–9.
Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM, et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant. 2007;13:655–64.
Armand P, Kim HT, Zhang MJ, Perez WS, Dal Cin PS, Klumpp TR, et al. Classifying cytogenetics in patients with acute myelogenous leukemia in complete remission undergoing allogeneic transplantation: a Center for International Blood and Marrow Transplant Research study. Biol Blood Marrow Transplant. 2012;18:280–8.
Chevallier P, Labopin M, Milpied N, Cornelissen JJ, Blaise D, Petersen E, et al. Impact of cytogenetics risk on outcome after reduced intensity conditioning allo-SCT from an HLA-identical sibling for patients with AML in first CR: a report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2012;47:1442–7.
Ferrant A, Labopin M, Frassoni F, Prentice HG, Cahn JY, Blaise D, et al. Karyotype in acute myeloblastic leukemia: prognostic significance for bone marrow transplantation in first remission: a European Group for Blood and Marrow Transplantation study. Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Blood. 1997;90:2931–8.
Ogawa H, Ikegame K, Kawakami M, Takahashi S, Sakamaki H, Karasuno T, et al. Impact of cytogenetics on outcome of stem cell transplantation for acute myeloid leukemia in first remission: a large-scale retrospective analysis of data from the Japan Society for Hematopoietic Cell Transplantation. Int J Hematol. 2004;79:495–500.
Alloin AL, Leverger G, Dalle JH, Galambrun C, Bertrand Y, Baruchel A, et al. Cytogenetics and outcome of allogeneic transplantation in first remission of acute myeloid leukemia: the French pediatric experience. Bone Marrow Transplant. 2017;52:516–21.
Chalandon Y, Barnett MJ, Horsman DE, Conneally EA, Nantel SH, Nevill TJ, et al. Influence of cytogenetic abnormalities on outcome after allogeneic bone marrow transplantation for acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2002;8:435–43.
Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010;116:1839–48.
Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–91.
Walter RB, Pagel JM, Gooley TA, Petersdorf EW, Sorror ML, Woolfrey AE, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010;24:1276–82.
Yanada M, Kurosawa S, Yamaguchi T, Uchida N, Miyawaki S, Kanamori H, et al. Effect of related donor availability on outcome of AML in the context of related and unrelated hematopoietic cell transplantation. Bone Marrow Transplant. 2013;48:390–5.
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310–6.
Bertaina A, Zecca M, Buldini B, Sacchi N, Algeri M, Saglio F, et al. Unrelated donor vs HLA-haploidentical alpha/beta T-cell- and B-cell-depleted HSCT in children with acute leukemia. Blood. 2018;132:2594–607.
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood. 2015;126:1033–40.
Berger M, Lanino E, Cesaro S, Zecca M, Vassallo E, Faraci M, et al. Feasibility and outcome of haploidentical hematopoietic stem cell transplantation with post-transplant high-dose cyclophosphamide for children and adolescents with hematologic malignancies: an AIEOP-GITMO retrospective multicenter study. Biol Blood Marrow Transplant. 2016;22:902–9.
Locatelli F, Merli P, Pagliara D, Li Pira G, Falco M, Pende D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood. 2017;130:677–85.
Mrozek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–23.
Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89.
Rollig C, Bornhauser M, Thiede C, Taube F, Kramer M, Mohr B, et al. Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29:2758–65.
Sharma A, Huang S, Li Y, Brooke RJ, Ahmed I, Allewelt HB, et al. Outcomes of pediatric patients with therapy-related myeloid neoplasms. Bone Marrow Transplant. 2021;56:2997–3007.
Bitan M, He W, Zhang MJ, Abdel-Azim H, Ayas MF, Bielorai B, et al. Transplantation for children with acute myeloid leukemia: a comparison of outcomes with reduced intensity and myeloablative regimens. Blood. 2014;123:1615–20.
Acknowledgements
We would like to thank Keith A. Laycock, PhD, ELS for scientific editing of the manuscript. We would like to thank our colleagues, advanced practice providers, nurses, data managers and other healthcare professional who participated in patient care. We would also like to thank the parents who entrusted the care of our children to us. This work was supported by funds from the American Lebanese Syrian Associated Charities (ALSAC) (to Akshay Sharma).
Author information
Authors and Affiliations
Contributions
AS, NSB and SVB conceptualized and designed the study, interpreted the results, wrote the first draft of the manuscript, and made revisions. J-EG and AD provided data management and performed statistical analysis. KK and SC oversaw the study and provided supervision. Several authors were involved in caring for the patients included in this study at their respective centers. All authors critically reviewed the manuscript, provided comments, and approved the final version.
Corresponding author
Ethics declarations
Competing interests
AS has received consultant fees from Spotlight Therapeutics, Medexus Inc., Vertex Pharmaceuticals, Sangamo Therapeutics, and Editas Medicine. He is a medical monitor for an RCI BMT CSIDE clinical trial for which he receives financial compensation. He has also received research funding from CRISPR Therapeutics and honoraria from Vindico Medical Education. AS is the St. Jude Children’s Research Hospital site principal investigator of clinical trials for genome editing of sickle cell disease sponsored by Vertex Pharmaceuticals/CRISPR Therapeutics (NCT03745287), Novartis Pharmaceuticals (NCT04443907), and Beam Therapeutics (NCT05456880). The industry sponsors provide funding for the clinical trial, which includes salary support paid to AS’s institution. AS has no direct financial interest in these therapies.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sharma, A., Galimard, JE., Pryce, A. et al. Cytogenetic abnormalities predict survival after allogeneic hematopoietic stem cell transplantation for pediatric acute myeloid leukemia: a PDWP/EBMT study. Bone Marrow Transplant 59, 451–458 (2024). https://doi.org/10.1038/s41409-024-02197-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02197-3