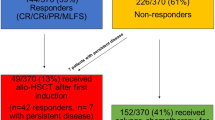
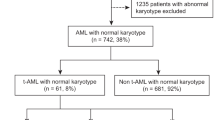
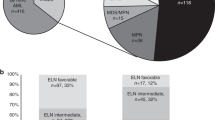
Abstract
Therapy-related myeloid neoplasms (t-MN) are aggressive myeloid neoplasms. Factors predicting post-allogeneic stem cell transplant (alloSCT) survival are not well-known. We studied the prognostic utility of factors at: t-MN diagnosis, pre-alloSCT, and post-alloSCT. Primary endpoints were 3-year overall survival (OS), relapse incidence (RI), and non-relapse mortality (NRM). Post-alloSCT OS did not differ between t-MDS and t-AML (20.1 vs. 19.6 months, P = 1), though t-MDS had a significantly higher 3-year RI compared to t-AML (45.1% vs. 26.9%, P = 0.03). In t-MDS, the presence of monosomy 5 (HR 3.63, P = 0.006) or monosomy 17 (HR 11.81, P = 0.01) pre-alloSCT were associated with higher RI. Complex karyotype was the only factor adversely influencing survival at all the timepoints. The inclusion of genetic information yielded 2 risk-categories: high-risk defined by the presence of pathogenic variants (PV) in (TP53/BCOR/IDH1/GATA2/BCORL1) and standard-risk (remainder of the patients) with 3-year post-alloSCT OS of 0% and 64.6%, respectively (P = 0.001). We concluded that while alloSCT was curative in a subset of t-MN patients, outcomes remained poor, specifically in the high-risk category. t-MDS patients, especially those with persistent disease pre-alloSCT were at increased risk of relapse. Disease-related factors at t-MN diagnosis were the most prognostic of post-alloSCT survival; utility of factors available later in the course, was incremental.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding authors upon reasonable request (devendra.hiwase@sa.gov.au or shah.mithun@mayo.edu).
References
Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–405.
Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, et al. Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study. J Clin Oncol. 2015;33:3641–9.
Metheny L, Callander NS, Hall AC, Zhang MJ, Bo-Subait K, Wang HL, et al. Allogeneic transplantation to treat therapy-related myelodysplastic syndrome and acute myelogenous leukemia in adults. Transpl Cell Ther. 2021;27:923.e1–923.e12.
McNerney ME, Godley LA, le Beau MM. Therapy-related myeloid neoplasms: when genetics and environment collide. Nat Rev Cancer. 2017;17:513–27.
Baranwal A, Hahn CN, Shah MV, Hiwase DK. Role of germline predisposition to therapy-related myeloid neoplasms. Curr Hematol Malig Rep. 2022;17:254–65.
Smith SM, le Beau MM, Huo D, Karrison T, Sobecks RM, Anastasi J, et al. Clinical-cytogenetic associations in 306 patients with therapy-related myelodysplasia and myeloid leukemia: the University of Chicago series. Blood. 2003;102:43–52.
Morton LM, Dores GM, Tucker MA, Kim CJ, Onel K, Gilbert ES, et al. Evolving risk of therapy-related acute myeloid leukemia following cancer chemotherapy among adults in the United States, 1975-2008. Blood. 2013;121:2996–3004.
Eichenauer DA, Thielen I, Haverkamp H, Franklin J, Behringer K, Halbsguth T, et al. Therapy-related acute myeloid leukemia and myelodysplastic syndromes in patients with Hodgkin lymphoma: a report from the German Hodgkin Study Group. Blood 2014;123:1658–64.
Nadiminti K, Sidiqi MH, Meleveedu K, Alkhateeb HB, Hogan WJ, Litzow M, et al. Characteristics and outcomes of therapy-related myeloid neoplasms following autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2021;11:63.
Chang C, Storer BE, Scott BL, Bryant EM, Shulman HM, Flowers ME, et al. Hematopoietic cell transplantation in patients with myelodysplastic syndrome or acute myeloid leukemia arising from myelodysplastic syndrome: similar outcomes in patients with de novo disease and disease following prior therapy or antecedent hematologic disorders. Blood. 2007;110:1379–87.
Fianchi L, Pagano L, Piciocchi A, Candoni A, Gaidano G, Breccia M, et al. Characteristics and outcome of therapy-related myeloid neoplasms: report from the Italian network on secondary leukemias. Am J Hematol. 2015;90:E80–5.
Litzow MR, Tarima S, Pérez WS, Bolwell BJ, Cairo MS, Camitta BM, et al. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–7.
Kroger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, et al. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94:542–9.
Ossenkoppele GJ, Janssen JJWM, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. 2016;101:20–5.
Singhal D, Wee LYA, Kutyna MM, Chhetri R, Geoghegan J, Schreiber AW, et al. The mutational burden of therapy-related myeloid neoplasms is similar to primary myelodysplastic syndrome but has a distinctive distribution. Leukemia. 2019;33:2842–53.
Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable (‘minimal’) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016;30:1456–64.
Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, et al. Early post-transplant minimal residual disease assessment improves risk stratification in acute myeloid leukemia. Biol Blood Marrow Transplant. 2018;24:1514–20.
Maffini E, Labopin M, Beelen DW, Kroeger N, Arat M, Wilson KMO, et al. Measurable residual disease (MRD) status before allogeneic hematopoietic cell transplantation impact on secondary acute myeloid leukemia outcome. A Study from the Acute Leukemia Working Party (ALWP) of the European society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transpl. 2022;57:1556–63.
Hulegårdh E, Nilsson C, Lazarevic V, Garelius H, Antunovic P, Rangert Derolf Å, et al. Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry. Am J Hematol. 2015;90:208–14.
Kayser S, Döhner K, Krauter J, Köhne CH, Horst HA, Held G, et al. The impact of therapy-related acute myeloid leukemia (AML) on outcome in 2853 adult patients with newly diagnosed AML. Blood. 2011;117:2137–45.
Finke J, Schmoor C, Bertz H, Marks R, Wäsch R, Zeiser R, et al. Long-term follow-up of therapy-related myelodysplasia and AML patients treated with allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2016;51:771–7.
Hiwase D, Hahn C, Tran ENH, Chhetri R, Baranwal A, Al-Kali A, et al. TP53 mutation in therapy-related myeloid neoplasm defines a distinct molecular subtype. Blood. 2023;141:1087–91.
Shah M, Tran ENH, Shah S, Chhetri R, Baranwal A, Ladon D, et al. TP53 mutation variant allele frequency of ≥10% is associated with poor prognosis in therapy-related myeloid neoplasms. Blood Cancer J. In press.
Nanaa A, Viswanatha D, Xie Z, Jevremovic D, Nguyen P, Salama ME, et al. Clinical and biological characteristics and prognostic impact of somatic GATA2 mutations in myeloid malignancies: a single institution experience. Blood Cancer J. 2021 ;11:122.
Wlodarski MW, Collin M, Horwitz MS. GATA2 deficiency and related myeloid neoplasms. Semin Hematol. 2017;54:81–6.
Feng JH, Guo XP, Chen YY, Wang ZJ, Cheng YP, Tang YM. Prognostic significance of IDH1 mutations in acute myeloid leukemia: a meta-analysis. Am J Blood Res. 2012;2:254–64.
Lachowiez CA, Reville PK, Kantarjian H, Jabbour E, Borthakur G, Daver N, et al. Contemporary outcomes in <scp> IDH </scp> ‐mutated acute myeloid leukemia: The impact of co‐occurring <scp> NPM1 </scp> mutations and venetoclax‐based treatment. Am J Hematol. 2022;97:1443–52.
Baranwal A, Shah MV, Barua A, Yikilmaz AS, He R, Viswanatha DS, et al. Characteristics and Outcomes of Patients with BCOR Mutated Myeloid Neoplasms. Blood. 2022;140:3235–6. (Supplement 1).
Terada K, Yamaguchi H, Ueki T, Usuki K, Kobayashi Y, Tajika K, et al. Usefulness of BCOR gene mutation as a prognostic factor in acute myeloid leukemia with intermediate cytogenetic prognosis. Genes Chromosomes Cancer. 2018;57:401–8.
Döhner H, Wei AH, Appelbaum FR, Craddock C, DiNardo CD, Dombret H, et al. Diagnosis and management of AML in adults: 2022 recommendations from an international expert panel on behalf of the ELN. Blood. 2022;140:1345–77.
Thiede C, Bornhäuser M, Oelschlägel U, Brendel C, Leo R, Daxberger H, et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia. 2001;15:293–302.
Preuner S, Peters C, Potschger U, Daxberger H, Fritsch G, Geyeregger R, et al. Risk assessment of relapse by lineage-specific monitoring of chimerism in children undergoing allogeneic stem cell transplantation for acute lymphoblastic leukemia. Haematologica. 2016;101:741–6.
Holtan SG, Hamadani M, WU J, Al Malki MM, Runaas L, Elmariah H, et al. Post-transplant cyclophosphamide, tacrolimus, and mycophenolate mofetil as the new standard for graft-versus-host disease (GVHD) prophylaxis in reduced intensity conditioning: results from phase III BMT CTN 1703. Blood. 2022;140(Supplement 2):LBA-4-LBA-4.
Baranwal A, Langer KJ, Ali K, Kharfan-Dabaja MA, Ayala E, Foran J, et al. Surrogates of endothelial injury predict non-relapse mortality among patients undergoing allogeneic hematopoietic stem cell transplantation with post-transplant cyclophosphamide-based gvhd prophylaxis. Transpl Cell Ther. 2023;29:S9–10.
Saha A, Blazar BR. Antibody based conditioning for allogeneic hematopoietic stem cell transplantation. Front Immunol. 2022;13. https://doi.org/10.3389/fimmu.2022.1031334
Hsu JI, Dayaram T, Tovy A, de Braekeleer E, Jeong M, Wang F, et al. PPM1D mutations drive clonal hematopoiesis in response to cytotoxic chemotherapy. Cell Stem Cell. 2018;23:700–713.e6.
Martin JE, Khalife-Hachem S, Grinda T, Kfoury M, Garciaz S, Pasquier F, et al. Therapy-related myeloid neoplasms following treatment with PARP inhibitors: new molecular insights. Ann Oncol. 2021;32:1046–8.
Khoury JD, Solary E, Abla O, Akkari Y, Alaggio R, Apperley JF, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 2022;36:1703–19.
Acknowledgements
We are grateful to our patients and their families. MVS was supported by Paul Calabresi Program in Clinical/Translational Research at Mayo Clinic (K12CA090628); Leukemia Research Foundation New Investigator Award; and Bridget Kiely Clinician Career Development in Transplant Research. DKH was supported by Investigator Grant, NHMRC/MRFF and Cancer Australia. BioRender software was used to create Fig. 1a.
Author information
Authors and Affiliations
Contributions
AB, MVS, and DKH designed the study; AB, RC, MC, DKH, and MVS collated the data; AB, RC, and CK performed the statistical analysis; AB, DY, MRL, WJH, AM, HBA, DS, AC, PB, DKH, and MVS contributed patients; AB drafted the manuscript and DKH and MVS edited the manuscript. Other authors edited the manuscript, and all agree to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
MRL—Research funding (AbbVie, Astellas, Amgen, Actinium, Pluristem), Advisory board (Jaz, Omeros), Data monitoring committee (BioSight); DY—Research funding from BMS and Novartis, Honoraria from Novartis, Pfizer, Amgen, and Takeda; DKH—Membership on an entity’s Board of Directors or advisory committees (AbbVie, Novartis); MVS—Research funding to the institution (Abbvie, Celgene, Astellas, and MRKR Therapeutics). The other authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baranwal, A., Chhetri, R., Yeung, D. et al. Factors predicting survival following alloSCT in patients with therapy-related AML and MDS: a multicenter study. Bone Marrow Transplant 58, 769–776 (2023). https://doi.org/10.1038/s41409-023-01970-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-023-01970-0