Abstract
With the advent of surfactant and gentle ventilation, the incidence of neonatal pneumothorax has decreased over the last two decades. Pneumothorax associated with respiratory distress syndrome is more common in preterm infants, but term infants often present with isolated pneumothorax. The use of CPAP or non-invasive respiratory support in the delivery room for a term infant with respiratory distress increases transpulmonary pressures and increases the risk of pneumothorax. Prompt diagnosis with a high index of suspicion, quick evaluation by transillumination, chest X-ray or lung ultrasound is critical. Management includes observation, needle thoracocentesis and if necessary, chest tube placement. This manuscript reviews the incidence, pathogenesis, diagnosis and management of a term infant with isolated pneumothorax, summarizing the combination of established knowledge with new understanding, including data on diagnostic modes such as ultrasound, reviewing preventative measures, and therapeutic interventions such as needle thoracocentesis and a comparison of pigtail vs. straight chest tubes.
Similar content being viewed by others
Introduction
Respiratory distress in the term neonate represents a large proportion of newborns who require admission to the neonatal intensive care unit (NICU) [1]. An underlying pulmonary disease such as meconium aspiration syndrome (MAS) or transient tachypnea of the newborn (TTN) [2, 3], which often requires positive pressure ventilation (PPV) or continuous positive airway pressure (CPAP), increases the risk of a pneumothorax [4]. Albeit, much less common, a spontaneous pneumothorax in an otherwise healthy newborn should always be included in the differential diagnosis of respiratory distress. Prompt recognition that a pneumothorax is causing or exacerbating respiratory distress is critical to improve outcomes and decrease morbidity and mortality related to this condition. The presence of pneumothorax significantly increases hospital length of stay (median 4 days, 95% confidence interval, 3.1–8.5 days) [5, 6]. The optimal means to prevent, diagnose, and manage pneumothoraces in term neonates have not yet been established and current evidence will be reviewed in this manuscript.
Incidence
A large retrospective study of 19 US hospitals evaluating respiratory morbidity included data on approximately 166,000 term infants (37 to 40-week gestational age-GA), which showed an incidence of symptomatic pneumothorax requiring NICU admission of 0.1% (159 neonates) [7]. Data from the Kids Inpatient Database (KID) from the US showed a prevalence of 0.02% among infants born at ≥37 weeks GA [6]. A Danish study including almost 49,000 neonates found a similar incidence of symptomatic pneumothorax in 34 term neonates (0.07%) with all except one patient having an underlying pulmonary disease (TTN in 62% and MAS in 10%) [8]. Delivery by cesarean section increases the risk of pneumothorax possibly due to increased risk of TTN [9]. In a subgroup analysis comparing term and preterm neonates with pneumothorax from a single center, the majority of term neonates presented with symptoms within 24 h after birth (98% in term infants compared to 70% in preterm) and recovered with a low frequency of invasive treatment (12% in term, 40% in preterm) [9]. These data suggest that pneumothorax is seen in 0.02 to 0.1% (or 1 in 1000 to 5000 term infants), and a high proportion of pneumothoraces in the term population are mild or asymptomatic.
Pathophysiology
The transpulmonary pressure gradient (Ptp) is the difference between alveolar pressure (Palv) and pleural pressure (Ppl) [10]. During normal breathing, the pressure in the pleural space is negative, and when the diaphragm contracts, it creates a negative pressure in the alveolar space, which pulls air into the lung and expands outward due to the surface tension between the parietal and visceral pleura [11]. Soon after birth, term infants generate high negative intrapleural pressure (–40 to –50 cm H2O) during the first breath [12]. Subsequently, in a normal spontaneously breathing newborn infant, diaphragmatic activity results in negative pleural pressure (e.g. –3 to –10 cm H2O, mean – 5 cm H2O) [13], and at the end of inspiration, the alveolar pressure equilibrates with atmospheric pressure and is 0 (Fig. 1A) resulting in a mean peak Ptp of 5 cm H2O. In a sedated or paralyzed endotracheally intubated patient on synchronized mechanical ventilation, the alveolar pressure is positive and so is the pleural pressure resulting in a small transpulmonary gradient (Fig. 1B). In a neonate in severe distress with increased work of breathing, a very high negative pleural pressure can be generated (Fig. 1C). When CPAP or other non-invasive positive pressure support is given, alveolar pressure is positive, resulting in an even higher transpulmonary gradient, which increases the risk of air-leak [10]. This situation is common in term infants in respiratory distress with retractions that can generate high negative pleural pressure while receiving mask CPAP in the delivery room [10]. In addition, parenchymal lung disease associated with expiratory flow obstruction is common in MAS and TTN due to airway narrowing from meconium or retained lung liquid, respectively (Fig. 1C and Fig. 2). This expiratory obstruction can create a ball-valve effect resulting in alveolar overdistension from further increase in transpulmonary pressures and rupture (Fig. 2).
Ptp is the difference between alveolar pressure (Palv) and pleural pressure (Ppl). During normal respiration (A), diaphragmatic contraction results in negative Ppl leading to inhalation. At the end of inhalation, Palv is the same as atmospheric pressure. During invasive mechanical ventilation in a sedated or paralyzed neonate, Palv and Ppl are both positive and Ptp is low (B). A newly born infant in respiratory distress has high negative Ppl. Providing delivery room CPAP (DR CPAP) or CPAP in the early neonatal period results in positive Palv. This leads to high Ptp increasing the risk for an air leak. C The presence of meconium aspiration or retained lung liquid leads to a ball-valve effect leading to expiratory obstruction and alveolar distension resulting in further elevation of Palv increasing the risk of air leak. Copyright Satyan Lakshminrusimha.
Unequal alveolar expansion at birth is further exacerbated by poorly developed pores of Kohn and canals of Lambert limiting ability to exchange air between alveoli. Ball-valve effect from expiratory obstruction by retained lung liquid or meconium leads to overdistension. Air leak secondary to increased transpulmonary pressure is tracked along vasculature leading to pneumomediastinum and pneumothorax. Copyright Satyan Lakshminrusimha.
The classical studies of Macklin have suggested that air after extrusion from the ruptured alveolus follows along the perivascular sheaths of the lung, either out to the visceral pleural surface and into the pleural cavity or will pass to the root of the lung, into the mediastinum and then through the mediastinal pleura into the pleural cavity [14]. This proposed mechanism suggests that pneumomediastinum and pneumothorax are part of the same air-leak spectrum in neonates. The lungs of the newborn infant expand rapidly, but not all alveoli expand at the same time [15, 16]. When a baby takes their first few breaths, their diaphragm can produce a high transpulmonary pressure (Fig. 2) [17]. However, if some of the bronchial or bronchiolar passages are obstructed by aspirated meconium or retained lung liquid, the high transpulmonary pressure can be exerted on a small number of expanded alveoli or groups of alveoli. In newborns, the pores of Kohn, Martin’s and Lambert’s channels and the intersegmental bronchioles, which are effective collateral channels in normal lungs, are not well developed. This makes it difficult for the high-pressure air in these groups of alveoli to pass easily into adjacent, unexpanded alveoli [14]. This high pressure can cause the overdistended alveoli to rupture, and air can escape into the perivascular sheaths and cause a pneumomediastinum or pneumothorax. Thus, foreign material aspirated into (e.g., meconium) or retained in the lungs (e.g., fetal lung fluid) of newborns can cause alveolar rupture due to high pressure in a small number of expanded alveoli, with air escaping into the pleural space directly or through the perivascular sheaths [18]. During respiration, the bronchi and bronchioles dilate with inhalation and contract with exhalation. A partial bronchial obstruction during inhalation becomes a complete obstruction in exhalation because the obstructing mass acts as a ball valve (Fig. 2). A partial obstruction allows air to pass into the distal alveoli during inhalation, but on exhalation the bronchus is completely obstructed, and air cannot be expelled. Consequently, the alveoli distend until they rupture. Ruptured alveoli on the pleural surface result in a pneumothorax. If pressure in the mediastinum is great enough, air may escape and track into the subcutaneous tissue of the neck and chest to cause subcutaneous emphysema.
A persistent air leak is one that exists for greater than five to seven days, but the incidence in a term neonate is unclear except for some postoperative patients with congenital anomalies of the airway or lung. It is more common in preterm neonates. In a small retrospective review of 42 term and preterm neonates with pneumothorax, risk factors for persistent pneumothorax or mortality included underlying primary lung pathology, need for mechanical ventilation, and bilateral pneumothorax [19].
Diagnosis
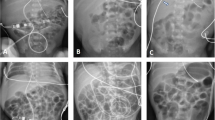
Pneumothorax should be considered within the differential diagnosis of every neonate who is showing signs of respiratory distress. A pneumothorax may be asymptomatic, symptomatic or under tension. Prompt diagnosis is critical as development of a tension pneumothorax can lead to acute hypoxia, respiratory failure, severe hemodynamic compromise and death. The most common clinical signs are respiratory distress associated with decreased air entry on the affected side when auscultating over the hemithorax. Diffuse rales and rhonchi may be appreciated throughout both lung fields. Rarely, bulging of the chest anteriorly may be noticed if a large collection of air remains in the mediastinum. A tension pneumothorax is when thoracic pressure is increased to a degree that it impedes venous return to the heart. When the pneumothorax is under tension, the patient may be tachycardic and the cardiac impulse may be displaced. Transillumination with a bright light applied to the chest can be helpful in making a diagnosis especially in preterm infants with a thin chest wall [20] but is not accurate in term infants [21] or preterm infants with a thicker chest wall. Traditionally, chest radiography has been the diagnostic imaging choice. Since most neonatal X-rays are taken with the infant supine on the warmer or incubator, and air is usually anterior, one border of the cardiac silhouette is usually sharper than the other (Fig. 3). With a large pneumothorax, mediastinal shift and a lung border with free air next to it may be visible (Fig. 3).
Recently, point-of-care ultrasound (POCUS) has been shown to have similar accuracy in detecting a pneumothorax compared to radiography with a shorter time to diagnosis [22]. Evidence-based POCUS guidelines [23] and crashing neonatal protocols (such as “Sonographic Assessment of life-threatening Emergencies—Revised” (SAFE-R)) protocol or crashing neonatal protocol (CNP) recommend use of POCUS for rapid diagnosis of pneumothorax in emergency situations [24]. The three cardinal signs of pneumothorax on lung ultrasound are: (1) absence of lung sliding, (2) absence of B-lines, (3) and presence of A lines (Fig. 4A, B) [25]. Lung sliding occurs when visceral and parietal pleura slide against each other to create the ultrasound feature. The absence of lung slide (which indicates air between the two pleural surfaces pretending direct contact). The “lung point” is seen at the junction of lung sliding (showing normal lung) and no lung sliding (showing pneumothorax) (Fig. 4A). When present, “lung point” is diagnostic of pneumothorax with 100% specificity (Fig. 4A, B). However, in a large tension pneumothorax, the lung point may not be identified as the transition zone between normally apposed pleura and pneumothorax does not exist. Lung POCUS can help in differentiating between small and large pneumothoraces, based on the location of the lung point indicating the extent of the pneumothorax. POCUS can’t be used to estimate the degree of overdistension in alveoli or quantify air-leak in pneumothorax [20]. Additional M-mode of “barcode” or “stratosphere” sign can further confirm pneumothorax, whereas normal lung appears differently as “seashore” sign in M-mode.
Management
Preventative measures
Continuous positive airway pressure use in the delivery room (DR CPAP) for term neonates has increased since recommendations changed to consider use in term and late preterm neonates by various governing bodies [26], which has resulted in a higher prevalence of various pulmonary air leak syndromes including pneumothorax [27]. In a large, nested cohort study, pneumothorax associated with DR CPAP was higher in neonates using 21% oxygen than those receiving supplemental oxygen, implying that caution should be applied for the use of CPAP in term and late preterm infants for transient grunting, nasal flaring and tachypnea in the delivery room [28]. Stocks et al. attempted to de-implement term and late preterm neonates from being exposed to DR CPAP administration, especially to neonates that did not need PPV or supplemental oxygen administration in the DR. This decrease in DR CPAP was achieved without a significant increase in other adverse outcomes, such as NICU admissions, and showed a decrease in DR related pneumothoraces [29].
Adult literature suggests that inhaling higher concentrations of oxygen improves resolution of a symptomatic pneumothorax [30, 31]. Such use of hyperoxia is based on the theory that the inhalation of 100% oxygen reduces the partial pressure of nitrogen in the alveolus compared to the pleural space. This gradient causes the nitrogen to diffuse from the pleural space into the alveoli, resulting in the reabsorption of air. Studies in term infants show that a nitrogen washout does not result in faster resolution of the pneumothorax and results in unnecessary exposure to increased oxygen [30, 31]. Furthermore, compared to nitrogen, oxygen has high solubility in blood and can quickly diffuse into the pulmonary vasculature. This can cause insufficient gas levels in the alveoli, resulting in alveolar collapse, especially if oxygen is delivered without pressure using an oxyhood (absorption atelectasis) [32]. Exposure to 100% oxygen has been associated with increased oxidative stress and pulmonary vascular hyperactivity [33, 34].
Some ventilatory strategies have been shown to improve the risk of pneumothorax. A Cochrane Review from 2017, there was a significant reduction in pneumothorax for the infants ventilated using volume targeted ventilation vs. pressure limited ventilation [35]. Ventilator breaths should also be synchronized as much as possible to the infant’s breaths as asynchrony can increase the risk of pneumothoraxes [5]. In preterm infants, high frequency oscillator ventilation (HFOV) has an increased risk of creating acute air leak [36]. However, some data suggests that high frequency ventilation may be protective against air leaks. Gonzalez et al. showed there was decreased gas flow through the chest tube after receiving high frequency jet ventilation (HFJV) [37]. Surfactant administration is most often used in the preterm population for respiratory distress syndrome, but it is also sometimes used in term infants, most commonly for meconium aspiration syndrome. There is thought to be some association between surfactant administration and risk for pneumothorax, but 2 small systematic reviews showed no difference in development of pneumothorax with surfactant [38]. The use of volume-targeted ventilation will minimize volutrauma with changing compliance following surfactant administration and potentially reduce the risk of air leak.
The mechanism through which HFJV ameliorates an air leak is not clear, but it is thought to be due to low tidal volumes that are otherwise unachievable via conventional ventilation. HFOV ventilation strategy using minimal mean airway pressure of 8-9 and high frequency (hertz of 10), as described in a case series of 5 preterm infants with unilateral pneumothorax, showed avoidance of increasing the air leak and avoiding progression of pneumothorax [39]. Such a strategy avoids the complication of chest tube insertion altogether in preterm infants. However, this approach may not be translatable to term neonates.
Needle thoracentesis vs. chest tube thoracostomy
Needle thoracentesis consists of aspirating air through a small hypodermic needle or angiocath, where the needle is inserted into the second or third intercostal space at the midclavicular line and aspirating via a syringe. With the advent of ultrasonography, it may be possible to locate the optimal site for needle insertion at the bedside. Chest tube thoracostomy is the placement of a catheter in the fourth to sixth intercostal space at the midaxillary line. This can be done via a straight or pigtail catheter using Seldinger’s technique. Usually, this tube is placed to water seal or to continuous low wall suction (Fig. 5). A recent Cochrane review cites two controlled trials comparing needle aspiration to chest tube drainage and concluded that there was insufficient evidence to establish the efficacy and safety of needle aspiration alone. However, there were no differences in mortality and needle aspiration reduces the need for chest tube placement [40]. A retrospective cohort of 189 term and preterm infants with pneumothorax showed that approximately 20% of neonates were able to be treated with needle thoracentesis alone and concluded that it was reasonable to try needle aspiration prior to inserting a chest tube [28]. Another study of a total of 76 patients showed that needle aspiration reduced the rate of chest tube insertion in symptomatic infants (by 55%) and that needle aspiration should be the initial method to evacuate the air from the pleural space [41]. Pneumothoraces resulting from respiratory distress syndrome are more likely to require chest tube drainage compared to pneumothorax resulting from TTN [8].
Straight chest tube vs. pigtail catheter
Pigtail catheters are a safe and effective alternative to traditional chest tubes and recent data has shown improved outcomes with pigtail catheters [42, 43]. One study looking at complications of percutaneous catheters showed that pigtails resulted in more immediate radiological success compared to traditional straight catheters [43]. No other significant differences were noted. However, Reed et al. in a case series, suggested that pigtail catheters, as a result of their curve or because of the guidewire, may be more responsible for thoracic organ perforation, especially when they are placed emergently [44]. Traditional teaching is to insert the needle 1 cm into the chest, and then insert the guidewire, but in neonates, the chest wall thickness is often less than 1 cm, 1 cm insertion can result in thoracic organ injury, especially in emergent settings. It is safer to use the 4th intercostal space (ICS) as the distance between the chest wall and the border of the heart is longer at the 4th ICS compared to the 5th and 6th ICS. It is also safer to insert the needle 0.5 cm rather than the full 1 cm.
Drainage sets
The chest drainage system usually consists of a water seal, suction control and a drainage collection chamber (Fig. 5). They are designed to allow air or fluid to be suctioned out of the pleural cavity, prevent backflow, and restore negative pressure in the pleural space to allow for the lung to re-expand. Most chest tubes with suction are based on a three-chamber system (Fig. 5). The drainage from the chest flows into the chest tube and into the first chamber which allows for measurements and recording. The middle chamber is the water seal and air leak chamber which allows for air to exit the pleural space and prevents air from reentering the pleural cavity on inhalation. The third chamber allows for controlled suction, where there is a connection via tubing from the wall source of suction to the suction control chamber. The wall source of suction is increased until there is gentle bubbling in the suction control chamber. The level of suction applied to the chest tube is adjusted on the drainage system, not the wall suction source, and is typically set between –10 and –20 cm H2O. Tidaling can occur when the patient’s pressure float ball near the bottom of the water seal column moves up and down with the baby’s respirations. It moves up with spontaneous inhalation, due to higher negative pleural pressure and down during spontaneous exhalation. If a patient is on positive pressure ventilation, the reverse occurs [45].
Emergency equipment and bedside considerations
Typical equipment kept at bedside in the event of failure of the chest tube drainage system are smooth or non-toothed clamps, as well as a sterile bottle of water that can be used as a makeshift water seal. After needle aspiration, if the neonate is still unstable and the decision is made to insert a chest tube, it is reasonable to keep the angiocatheter used for the thoracentesis attached to a sterile bottle of water as a makeshift water seal, as the team is preparing for chest tube insertion. Petroleum gauze is also kept at the bedside and can be used as an occlusive dressing over the insertion site of inadvertent, premature removal of a chest tube to prevent entrainment of air into the pleural space in a spontaneously breathing infant.
Routine assessment of the drainage system is important. In addition to assessing output in the collection chamber, monitoring of the water seal and air leak chamber is imperative to assess the overall function of the system. While intermittent gentle bubbling in the water seal is indicative of an air leak in the patient, continuous bubbling in the water seal could reflect a bronchopleural fistula. Continuous bubbling could also demonstrate an air leak in the drainage system and corrective action must be taken. This could be secondary to an extrathoracic location of one of the side ports of the chest tube or a connection tube site leak.
Removal of the chest tube
Resolution of pneumothorax by chest X-ray or ultrasound and lack of active bubbling from the chest tube are indications for removal of the chest tube, as they indicate resolution of the pneumothorax without ongoing air leak. At this point, clinicians will often put the chest tube to water seal to determine if the pneumothorax reaccumulates. Some centers clamp the chest tube for a short period to check for reaccumulation, but this practice can be associated with development of a tension pneumothorax if there is a persistent air leak. Currently, the data comes from adult studies that show it appears to be safe and may be extrapolated to the neonatal population [46].
Long term consequences
Most term neonates have good outcomes following a pneumothorax. However, pneumothorax (especially one requiring intervention) has been shown to require a longer duration of hospital stay [40]. A delay in early recognition and management can be associated with significant mortality [47]. In a Danish study with 71 cases of symptomatic pneumothoraces, one third of the infants that had TTN required drainage [8]. Complications of chest tube placement to evacuate a pneumothorax include chest injury as well as breast scarring [48]. However, overall, symptomatic spontaneous pneumothorax in the full-term infant seems to be a relatively benign entity with spontaneous resolution and few long-term consequences [49].
Conclusion
Pneumothorax should be considered in the differential diagnosis in any neonate with respiratory distress. In term infants, it is more likely to be associated with TTN or MAS in which the retained fluid or meconium can create a ball-valve effect causing alveolar overdistension and eventual rupture. DR CPAP can also increase the risk of pneumothorax. The pores of Kohn which allow passage of air between alveoli are reduced in infants, and this does not allow for the air to pass to adjacent alveoli, creating uneven distension. X-ray remains the current diagnostic modality in most institutions, but point-of-care ultrasound is becoming more widely used to diagnose pneumothorax. Often, the neonate can be observed and does not need intervention. Needle thoracentesis may be used as a first line intervention and is associated with decreased rates of chest tube placement. Pigtail chest tubes have been shown to be as effective as standard straight chest tubes, with potentially fewer complications. Overall, pneumothorax in a term neonate, if identified promptly, has few consequences other than a longer length of hospital stay. As with much else in neonatology, further study must be done for several aspects of term neonates with pneumothorax, such as the role of ultrasound vs. chest X-ray in confirming the diagnosis, therapeutic interventions such as needle aspiration in various disease processes, best practices on removal of chest tubes, instances in which straight catheters or pigtail catheters are preferred, and more.
The manuscript has not been previously published and is not under consideration by any other publishing journal. All authors have contributed to the creation of this manuscript and approved of its contents. All authors have agreed to the Journal of Perinatology submission policies and have no conflicts of interest to declare. Funding for this review article was provided by NIH Grant RO1 grant.
References
Schulman J. Inborn NICU admission rates. The Problem of Practice Variation in Newborn Medicine: Critical Insights for Evaluating and Improving Quality. 1. Springer International Publishing; 2022. p. 145–52.
Guglani L, Lakshminrusimha S, Ryan RM. Transient tachypnea of the newborn. Pediatr Rev/Am Acad Pediatr. 2008;29:e59–65.
Alhassen Z, Vali P, Guglani L, Lakshminrusimha S, Ryan RM. Recent advances in pathophysiology and management of transient tachypnea of newborn. J Perinatol. 2021;41:6–16.
Kresmery P. Pneumothorax in the newborn. Neonatal Netw. 2000;19:62–53.
Greenough A, Rossor TE, Sundaresan A, Murthy V, Milner AD. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database Syst Rev. 2016;9:Cd000456.
Acun C, Nusairat L, Kadri A, Nusairat A, Yeaney N, Abu Shaweesh J, et al. Pneumothorax prevalence and mortality per gestational age in the newborn. Pediatr Pulmonol. 2021;56:2583–8.
Consortium on Safe L, Hibbard JU, Wilkins I, Sun L, Gregory K, Haberman S, et al. Respiratory morbidity in late preterm births. J Am Med Assoc. 2010;304:419–25.
Vibede L, Vibede E, Bendtsen M, Pedersen L, Ebbesen F. Neonatal pneumothorax: a descriptive regional Danish study. Neonatology. 2017;111:303–8.
Benterud T, Sandvik L, Lindemann R. Cesarean section is associated with more frequent pneumothorax and respiratory problems in the neonate. Acta Obstet Gynecol Scand. 2009;88:359–61.
Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–36.
McKnight CL, Burns B Pneumothorax. StatPearls: Treasure Island (FL), 2023.
Karlberg P, Koch G. Respiratory studies in newborn infants. III. Development of mechanics of breathing during the first week of life. A longitudinal study. Acta Paediatr Suppl (Upps). 1962;135:121–9.
Cook CD, Cherry RB, O’Brien D, Karlberg P, Smith CA. Studies of respiratory physiology in the newborn infant. I. Observations on normal premature and full-term infants. J Clin Investig. 1955;34:975–82.
Macklin CC. Transport of air along sheaths of pulmonic blood vessels from alveoli to mediastinum: clinical implications. Arch Intern Med. 1939;64:913–26.
Tingay DG, Farrell O, Thomson J, Perkins EJ, Pereira-Fantini PM, Waldmann AD, et al. Imaging the respiratory transition at birth: unraveling the complexities of the first breaths of life. Am J Respiratory Crit Care Med. 2021;204:82–91.
Lakshminrusimha S, Jobe AH. Baby’s first cries and establishment of gas exchange in the lung. Am J Respiratory Crit Care Med. 2021;204:11–13.
Agostoni E, Taglietti A, Agostoni AF, Setnikar I. Mechanical aspects of the first breath. J Appl Physiol. 1958;13:344–8.
Ashmore PG. Spontaneous pneumothorax in the newborn. Can Med Assoc J. 1965;92:309–11.
Esme H, Doğru O, Eren S, Korkmaz M, Solak O. The factors affecting persistent pneumothorax and mortality in neonatal pneumothorax. Turk J Pediatr. 2008;50:242–6.
Kuhns LR, Bednarek FJ, Wyman ML, Roloff DW, Borer RC. Diagnosis of pneumothorax or pneumomediastinum in the neonate by transillumination. Pediatrics. 1975;56:355–60.
Wyman ML, Kuhns LR. Accuracy of transillumination in the recognition of pneumothorax and pneumomediastinum in the neonate. Clin Pediatr. 1977;16:323–4.
Raimondi F, Rodriguez Fanjul J, Aversa S, Chirico G, Yousef N, De Luca D, et al. Lung ultrasound for diagnosing pneumothorax in the critically Ill neonate. J Pediatr. 2016;175:74–78 e71.
Singh Y, Tissot C, Fraga MV, Yousef N, Cortes RG, Lopez J, et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit Care. 2020;24:65.
Yousef N, Singh Y, De Luca D. “Playing it SAFE in the NICU” SAFE-R: a targeted diagnostic ultrasound protocol for the suddenly decompensating infant in the NICU. Eur J Pediatr. 2022;181:393–8.
Himebauch AS, Nishisaki A Basic lung ultrasound for the intensivist. In: Singh Y, Tissot C, Fraga MV, Conlon T (eds). Point-of-care ultrasound for the neonatal and pediatric intensivist. Springer Link: London UK, 2023.
Aziz K, Lee HC, Escobedo MB, Hoover AV, Kamath-Rayne BD, Kapadia VS, et al. Part 5: Neonatal Resuscitation: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142:S524–S550.
Hishikawa K, Goishi K, Fujiwara T, Kaneshige M, Ito Y, Sago H. Pulmonary air leak associated with CPAP at term birth resuscitation. Arch Dis Child Fetal Neonatal Ed. 2015;100:F382–387.
Smithhart W, Wyckoff MH, Kapadia V, Jaleel M, Kakkilaya V, Brown LS, et al. Delivery room continuous positive airway pressure and pneumothorax. Pediatrics. 2019;144:e20190756.
Stocks EF, Jaleel M, Smithhart W, Burchfield PJ, Thomas A, Mangona KLM, et al. Decreasing delivery room CPAP-associated pneumothorax at >/=35-week gestational age. J Perinatol. 2022;42:761–8.
Shaireen H, Rabi Y, Metcalfe A, Kamaluddeen M, Amin H, Akierman A, et al. Impact of oxygen concentration on time to resolution of spontaneous pneumothorax in term infants: a population based cohort study. BMC Pediatr. 2014;14:208.
Clark SD, Saker F, Schneeberger MT, Park E, Sutton DW, Littner Y. Administration of 100% oxygen does not hasten resolution of symptomatic spontaneous pneumothorax in neonates. J Perinatol. 2014;34:528–31.
O’Brien J. Absorption atelectasis: incidence and clinical implications. AANA J. 2013;81:205–8.
Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC 3rd, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59:137–41.
Lakshminrusimha S, Russell JA, Gugino SF, Ryan RM, Mathew B, Nielsen LC, et al. Adjacent bronchus attenuates pulmonary arterial contractility. Am J Physiol Lung Cell Mol Physiol. 2006;291:L473–478.
Klingenberg C, Wheeler KI, McCallion N, Morley CJ, Davis PG. Volume-targeted versus pressure-limited ventilation in neonates. Cochrane Database Syst Rev. 2017;10:Cd003666.
Cools F, Offringa M, Askie LM. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2015:CD000104.
Gonzalez F, Harris T, Black P, Richardson P. Decreased gas flow through pneumothoraces in neonates receiving high-frequency jet versus conventional ventilation. J Pediatr. 1987;110:464–6.
Ng EH, Shah V. Guidelines for surfactant replacement therapy in neonates. Paediatr Child Health. 2021;26:35–49.
Aurilia C, Ricci C, Tana M, Tirone C, Lio A, Gambacorta A, et al. Management of pneumothorax in hemodynamically stable preterm infants using high frequency oscillatory ventilation: report of five cases. Ital J Pediatr. 2017;43:114.
Joshi A, Kumar M, Rebekah G, Santhanam S. Etiology, clinical profile and outcome of neonatal pneumothorax in tertiary care center in South India: 13 years experience. J Matern-fetal Neonatal Med. 2022;35:520–4.
Murphy MC, Heiring C, Doglioni N, Trevisanuto D, Blennow M, Bohlin K, et al. Effect of needle aspiration of pneumothorax on subsequent chest drain insertion in newborns: a randomized clinical trial. JAMA Pediatr. 2018;172:664–9.
Wei YH, Lee CH, Cheng HN, Tsao LT, Hsiao CC. Pigtail catheters versus traditional chest tubes for pneumothoraces in premature infants treated in a neonatal intensive care unit. Pediatr Neonatol. 2014;55:376–80.
Panza R, Prontera G, Ives KN, Zivanovic S, Roehr CC, Quercia M, et al. Pigtail catheters versus traditional chest drains for pneumothorax treatment in two NICUs. Eur J Pediatr. 2020;179:73–79.
Reed RC, Waters BL, Siebert JR. Complications of percutaneous thoracostomy in neonates and infants. J Perinatol. 2016;36:296–9.
Zisis C, Tsirgogianni K, Lazaridis G, Lampaki S, Baka S, Mpoukovinas I, et al. Chest drainage systems in use. Ann Transl Med. 2015;3:43.
Chan YH, Yu EL, Kwok HC, Yeung YC, Yu WC. Clamping of chest drain before removal in spontaneous pneumothorax. J Cardiothorac Surg. 2021;16:24.
Basheer F, Aatif M, Saeed MHB, Jalil J. Clinical profile and outcome of neonatal pneumothorax in resource-limited neonatal intensive care unit. J Matern-fetal Neonatal Med. 2022;35:3373–8.
Jovandaric MZ, Milenkovic SJ, Dotlic J, Babovic IR, Jestrovic Z, Milosevic B, et al. Neonatal pneumothorax outcome in preterm and term newborns. Med (Kaunas). 2022;58:965.
Al Tawil K, Abu-Ekteish FM, Tamimi O, Al Hathal MM, Al Hathlol K, Abu Laimun B. Symptomatic spontaneous pneumothorax in term newborn infants. Pediatr Pulmonol. 2004;37:443–6.
Funding
The manuscript has not been previously published and is not under consideration by anyother publishing journal. All authors have agreed to the Journal of Perinatology submissionpolicies and have no conflicts of interest to declare. Funding for this review article was providedby NIH Grant RO1 grant. Funded by HD072929 (SL).
Author information
Authors and Affiliations
Contributions
VSJ, PV, SL contributed to teh conception and design and the first draft. YS contributed to the section on the use of ultrasound in the first draft as well as revisions. EG contributed to the section on setup in the first draft as well as revisions. VSJ, PV, and SL all revised the article. SL created the figures. All authors have contributed to the creation of this manuscript and approved of its contents.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jhaveri, V., Vali, P., Giusto, E. et al. Pneumothorax in a term newborn. J Perinatol 44, 465–471 (2024). https://doi.org/10.1038/s41372-024-01899-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-024-01899-2