Abstract
Objective
Despite limited safety and efficacy data, inhaled corticosteroids (ICS) are prescribed to premature infants in the neonatal intensive care unit (NICU). We examined contemporary use and risk factors for ICS use in the NICU.
Study design
Infants <33 weeks gestational age and <1500 gm birth weight discharged from Pediatrix Medical Group NICUs between 2010 and 2020 were included. We evaluated the association between ICS prescription and clinical characteristics using univariable and multivariable logistic regression.
Results
Of 74,123 infants from 308 NICUs, 9253 (12.5%) were prescribed ICS: budesonide, fluticasone, or beclomethasone. Diagnosis of bronchopulmonary dysplasia (BPD), earlier gestational age, male sex, longer mechanical ventilation, oxygen support, and systemic steroids were independent risk factors for ICS prescription.
Conclusions
Use of ICS is common in many NICUs and is associated with a diagnosis of BPD and healthcare utilization. Prospective trials are needed to establish the safety, efficacy, and optimal indication in this vulnerable population.
Similar content being viewed by others
Introduction
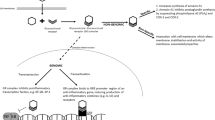
Inhaled corticosteroids (ICS) have been commonly prescribed to premature infants in the neonatal intensive care unit (NICU) [1,2,3,4,5]. However, ICS are not currently approved by The Food and Drug Administration (FDA) for use in children <12 months of age and are only currently approved for the maintenance treatment of asthma. The benefits of ICS in asthma may be limited to those with eosinophilic inflammation compared to those with other types of inflammation [6,7,8].
Previous studies of ICS use in the neonatal population have focused on the use of ICS in bronchopulmonary dysplasia (BPD) [1,2,3,4,5]. BPD is characterized by impaired lung development and is one of the leading causes of prematurity-related morbidity and mortality [9]. With the introduction of antenatal steroids and intratracheal surfactant, the morbidity and mortality rate of neonates with BPD is improving; however, there are still no U.S. FDA-approved drug therapies for infants to prevent or treat BPD [9, 10]. Studies supporting the use of ICS for BPD have been equivocal, and the long-term effects in premature infants are unknown (Supplemental Table 1) [11,12,13,14,15,16,17,18]. Currently there is insufficient evidence for expert organizations to recommend the routine use of ICS to prevent BPD or to treat the chronic respiratory symptoms of BPD [19, 20]. The objective of this study was to describe the contemporary use of ICS in NICUs across the US and to describe the risk factors for increased use.
Methods
This was a cohort study of premature infants discharged between 2010 and 2020 from a NICU in the Pediatrix Medical Group, Inc. The Pediatrix Medical Group, Inc prospectively captures information on infants cared for in NICUs from 35 states and Puerto Rico [21]. Inclusion and exclusion criteria were chosen to identify infants with complete hospitalizations and associated medication exposure histories. This study with deidentified data was reviewed and determined to be exempt from IRB review by the Duke University IRB (Pro0092987).
Subjects
We included infants with gestational age <33 weeks, birth weight <1500 g, and discharged after 14 days of age. Infants were excluded if they were <22 weeks gestation, not born in the hospital, transferred to another hospital, died during their hospitalization, or had a postmenstrual age (PMA) at discharge <33 weeks or >63 weeks to eliminate outliers.
Definitions
Demographic variables of interest included gestational age, birth weight, sex, race/ethnicity, and postmenstrual age at discharge. Clinical variables of interest included delivery mode, respiratory diagnoses, respiratory support, and medications of interest. Respiratory diagnoses of interest included physician diagnosed persistent pulmonary hypertension in the neonate (PPHN), pneumonia, aspiration, reactive airway disease, asthma, bronchiolitis, wheezing, tracheomalacia, bronchomalacia, chronic pulmonary insufficiency, BPD, and chronic lung disease. We also used a study definition for BPD, which was requiring supplemental oxygen, nasal cannula, continuous positive airway pressure, conventional mechanical ventilation, or high-frequency ventilation in infants from a PMA of 36 0/7 to 36 6/7 weeks [22]. Medications of interest included exposure to antenatal steroids, use of surfactant, montelukast, systemic steroids (including dexamethasone, hydrocortisone, prednisolone, and prednisone), diuretics (including acetazolamide, bumetanide, chlorothiazide, diazoxide, ethacrynic acid, furosemide, hydrochlorothiazide, metolazone, prazosin, and spironolactone), sildenafil, and bronchodilators (including albuterol, ipratropium). Respiratory support included use of mechanical ventilation and supplemental oxygen defined as fraction of inspired oxygen > 21% during their hospitalization. Individual NICU characteristics included annual total and BPD discharges and median gestational age.
Statistical analysis
We used standard summary statistics, including medians (25th–75th percentiles) and counts (percentages) to describe continuous and categorical study variables, including duration of ICS prescription. We also determined the prevalence of ICS prescription by specific drug over time. The primary outcome variable (ICS prescription ever) was analyzed using univariable and multivariable logistic regression. We included presence of the study definition of BPD, gestational age, antenatal steroid use, duration (by week) of oxygen support, mechanical ventilation, and postnatal systemic steroid, race/ethnicity, and sex as fixed effects in the multivariable model. Site was included as a random intercept. The strengths of associations were estimated using odds ratios (ORs) and 95% confidence intervals (CIs). Variables were included in the multivariable logistic regression with backwards selection using variables with p < 0.2 identified by univariable analysis.
Results
A total of 74,123 infants at 308 sites were included in the analyses, with 9253 (12.5%) infants prescribed ICS. Over the 11-year study period, annual ICS prescription has increased (Fig. 1). Budesonide was the most prescribed ICS overall and annually since 2012 (n = 5775, 7.8%), followed by fluticasone (n = 3529, 4.8%), and beclomethasone (n = 1255, 1.7%). There was considerable variation in ICS prescription by site. Among sites with at least 20 infants, the percentage of infants receiving ICS at each site ranged from 0 to 12% and 148 (65.8%) of sites did not prescribe ICS to any infants. Prescription of ICS in the NICU started at a median of 35 days (25th–75th percentile 21–56) at a median PMA 31 weeks (25th–75th percentile 29–34), ended at a median 69 days (25th–75th percentile 47–92) at a median PMA 36 weeks (25th–75th percentile 33–39), and for a median duration of 21 days (25th–75th percentile 5–44). ICS-prescribed infants were more likely to be male, have lower birthweight and gestational age than infants not prescribed ICS (Table 1). A study-defined diagnosis of BPD was made in 69% (n = 6358) of those prescribed ICS. Infants prescribed ICS also had a greater proportion diagnosed with other chronic respiratory conditions than infants not prescribed ICS. There were 584 (6%) infants prescribed ICS who did not have any of the respiratory diagnoses of interest documented. Infants prescribed ICS had a greater proportion receiving oxygen support and mechanical ventilation and for a longer duration than infants not prescribed ICS. Infants prescribed ICS were more likely to receive surfactant, diuretics, systemic steroids, and bronchodilators than infants not prescribed ICS. Additionally, infants prescribed ICS were born at NICUs with higher annual total and BPD discharges than infants not prescribed ICS.
In univariate logistic regression analyses, factors associated with an increase in the odds of ICS prescription included male sex, Black race, earlier gestational age, BPD diagnosis, and longer duration of supplemental oxygen, mechanical ventilation, and postnatal systemic steroids (Table 2). Multivariate logistic regression identified male sex, earlier gestational age, BPD diagnosis, duration of oxygen support, mechanical ventilation, and postnatal systemic steroid use as independent risk factors for ICS prescription.
Discussion
This study of premature infants born <33 weeks showed that ICS is prescribed off-label with regularity at many NICUs in the US. Diagnosis of BPD, male sex, gestational age, and healthcare utilization predicted ICS prescription. There was variability in ICS prescription by site, but the annual number of total and BPD discharges was associated with an increased odds of ICS prescription. We saw no evidence of a decline in use over the decade under study of this off-label therapy, suggesting the high need for improve therapies to prevent and treat BPD.
Previous analyses were limited to surveys of providers or populations of infants with BPD (Supplemental Table 1) [1,2,3,4,5]. We examined ICS use independent of diagnosis as the Pediatrix dataset captures prescription medications and diagnoses separately. Most infants prescribed ICS met the standardized criteria for BPD diagnosis, and the most common provider diagnosed conditions were chronic pulmonary insufficiency, chronic lung disease, and BPD. Due to the retrospective nature of our study, it is possible that some infants had BPD not captured by our definition, incomplete documentation, or another physician determined indication. This study corroborated real-world ICS use in infants <33 weeks predominantly in those either diagnosed or meeting criteria for BPD.
Oxygen support, mechanical ventilation, and postnatal systemic steroids were associated with ICS prescription. Previous studies have shown mixed efficacy of ICS for short-term benefits including earlier extubation, reduced supplemental oxygen need, and less systemic steroid use (Supplemental Table 1) [11,12,13,14,15,16,17,18, 23]. However, long term safety is not well established and the largest follow-up study found infants given inhaled budesonide had a higher mortality rate at 2 years than those given placebo [12]. Additionally, systematic reviews are challenging given the significant heterogeneity in the studies for the use of ICS for BPD. The studies were designed for different indications (prevention vs. treatment), patient populations, drugs (beclomethasone, budesonide, or fluticasone) at different doses, administration timing, and durations. The variability in ICS prescription is likely related to the lack of FDA guidance on prescription of ICS, and the limited options for neonatologists for treating severe respiratory failure and BPD.
We were unable to assess ICS use after discharge, but a longitudinal analysis of 130 children with severe BPD found sustained use of ICS in 35–40% from NICU discharge to 2 years of age [24]. The Bronchopulmonary Dysplasia Collaborative, a consortium of 12 US institutions studying BPD, currently recommends treating reactive small airway disease similarly to childhood asthma with ICS [25]. In their review, the expert group also discusses the paucity of data in these patients and recommends further evaluation to determine the appropriate dose, time, and type of steroid.
Current off-label use of ICS in the NICU is substantial and associated with a diagnosis of BPD, duration of respiratory support, and medication utilization. Using drugs off label and without high quality studies exposes infants to drugs that might not work and may be harmful. Future research is needed to determine not only the efficacy and safety of ICS for prevention of BPD, treatment of BPD, and treatment of asthma symptoms in children with a history of BPD, but also the dosing, timing, and type of ICS best for these at-risk populations.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Williams O, Greenough A. Post-natal corticosteroid use. Eur J Pediatr. 2003;162:613–5.
Porter KB, Garner SS. Survey of the use of corticosteroids among neonatal intensive care units for the prevention or treatment of bronchopulmonary dysplasia. J Pediatr Pharm Ther. 2005;10:90–9.
Maas C, Poets CF, Bassler D. Survey of practices regarding utilization of inhaled steroids in 223 German neonatal units. Neonatology. 2010;98:404–8.
Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Utilization of inhaled corticosteroids for infants with bronchopulmonary dysplasia. PLoS ONE. 2014;9:e106838.
Yao S, Uthaya S, Gale C, Modi N, Battersby C, UK Neonatal Collaborative (UKNC). Postnatal corticosteroid use for prevention or treatment of bronchopulmonary dysplasia in England and Wales 2012–2019: a retrospective population cohort study. BMJ Open. 2022;12:e063835.
Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor DR. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65:384–90.
Demarche S, Schleich F, Henket M, Paulus V, Louis R, Van Hees T. Step-down of inhaled corticosteroids in non-eosinophilic asthma: a prospective trial in real life. Clin Exp Allergy. 2018;48:525–35.
Lazarus SC, Krishnan JA, King TS, Lang JE, Blake KV, Covar R, et al. Mometasone or tiotropium in mild asthma with a low sputum eosinophil level. N Engl J Med. 2019;380:2009–19.
Voynow JA. ‘New’ bronchopulmonary dysplasia and chronic lung disease. Paediatr Respir Rev. 2017;24:17–18.
Zysman-Colman Z, Tremblay GM, Bandeali S, Landry JS. Bronchopulmonary dysplasia - trends over three decades. Paediatr Child Health. 2013;18:86–90.
Bassler D, Plavka R, Shinwell ES, Hallman M, Jarreau PH, Carnielli V, et al. Early inhaled budesonide for the prevention of bronchopulmonary dysplasia. N Engl J Med. 2015;373:1497–506.
Bassler D, Shinwell ES, Hallman M, Jarreau PH, Plavka R, Carnielli V, et al. Long-term effects of inhaled budesonide for bronchopulmonary dysplasia. N Engl J Med. 2018;378:148–57.
Clouse BJ, Jadcherla SR, Slaughter JL. Systematic review of inhaled bronchodilator and corticosteroid therapies in infants with bronchopulmonary dysplasia: implications and future directions. PLoS ONE. 2016;11:e0148188.
Shinwell ES, Portnov I, Meerpohl JJ, Karen T, Bassler D. Inhaled corticosteroids for bronchopulmonary dysplasia: a meta-analysis. Pediatrics. 2016;138:e20162511.
Shah VS, Ohlsson A, Halliday HL, Dunn M. Early administration of inhaled corticosteroids for preventing chronic lung disease in very low birth weight preterm neonates. Cochrane Database Syst Rev. 2017;1:CD001969.
Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for the treatment of bronchopulmonary dysplasia in ventilated very low birth weight preterm infants. Cochrane Database Syst Rev. 2017;10:CD002057.
Shah SS, Ohlsson A, Halliday HL, Shah VS. Inhaled versus systemic corticosteroids for preventing bronchopulmonary dysplasia in ventilated very low birth weight preterm neonates. Cochrane Database Syst Rev. 2017;10:CD002058.
Onland W, Offringa M, van Kaam A. Late (≥ 7 days) inhaled corticosteroids to reduce bronchopulmonary dysplasia in preterm infants. Cochrane Database Syst Rev. 2022;12:CD002311.
Allen J, Zwerdling R, Ehrenkranz R, Gaultier C, Geggel R, Greenough A, et al. Statement on the care of the child with chronic lung disease of infancy and childhood. Am J Respir Crit Care Med. 2003;168:356–96.
Watterberg KL, American Academy of Pediatrics. Committee on Fetus and Newborn. Policy statement-postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics. 2010;126:800–8.
Spitzer AR, Ellsbury DL, Handler D, Clark RH. The pediatrix BabySteps data warehouse and the pediatrix qualitysteps improvement project system-tools for ‘meaningful use’ in continuous quality improvement. Clin Perinatol. 2010;37:49–70.
Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M, et al. Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163:955–60.e1.
Nelin LD, Logan JW. The use of inhaled corticosteroids in chronically ventilated preterm infants. Semin Fetal Neonatal Med. 2017;22:296–301.
Akangire G, Manimtim W, Nyp MF, Noel-MacDonnell J, Kays AN, Truog WE, et al. Clinical outcomes among diagnostic subgroups of infants with severe bronchopulmonary dysplasia through 2 years of age. Am J Perinatol. 2018;35:1376–87.
Abman SH, Collaco JM, Shepherd EG, Keszler M, Cuevas-Guaman M, Welty SE, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr. 2017;181:12–28.e1.
Funding
NIH T32 AI007062-40. NIH R25 HD076475-06. NIH K24 HL143283.
Author information
Authors and Affiliations
Contributions
MT conceptualized the design, interpreted the data, and composed the initial draft of the manuscript. AI, CL, KM, AT, and DT participated in the design of the study and revised the manuscript. RK, RGG, and RHC acquired, analyzed, and interpreted the data, and revised the manuscript. CPH, KZ, MML participated in the design of the study, interpreted the data, and revised the manuscript. JEL conceptualized the design, interpreted the data, and revised the manuscript. All authors approved the final draft of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, M., Ibrahim, A., Laughon, C. et al. Prescribing practices of inhaled corticosteroids for premature infants in the neonatal intensive care unit. J Perinatol (2024). https://doi.org/10.1038/s41372-024-01891-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-024-01891-w