Abstract
Objective
To determine clinical, demographic, and hospital factors associated with inhaled bronchodilator (IB) use in infants with bronchopulmonary dysplasia (BPD) and specifically severe BPD.
Study design
Retrospective multicenter cohort study of 4986 infants born <32 weeks gestation with developing BPD at 28 days of life. We used the Pediatric Health Information System database to compare hospital experience and the demographic and clinical characteristics of infants exposed and not exposed to IBs.
Results
Twenty-five percent of BPD patients (1224/4986) and 48% of severe BPD patients (664/1390) received IBs. IB exposure was higher in infants with the tracheostomy, prolonged steroid and diuretic exposure, and longer duration of respiratory support. IB use varied markedly between hospitals (0–59%). Average annual BPD census was not associated with IB use.
Conclusion
Bronchodilator exposure is common in BPD patients with substantial variability in its use. Hospital experience did not account for the between-hospital variation in practice.
Similar content being viewed by others
Introduction
Bronchopulmonary dysplasia (BPD) is a chronic lung disease affecting an estimated 10,000–15,000 premature infants annually in the United States [1, 2]. It is associated with long-term lasting effects including small airway dysfunction, re-hospitalization for respiratory symptoms, increased need for respiratory medications into young adulthood, neurologic impairment, and mortality [1, 3,4,5,6]. Although no US Food and Drug Administration approved indication exists for the use of bronchodilators in the prevention or treatment of BPD, inhaled bronchodilators (IBs) are widely used in the neonatal intensive care unit (NICU) [7].
Inhaled bronchodilators are used to relax the smooth muscle in the bronchi and bronchioles, resulting in dilation of the small airways and potentially decreased airway resistance and increased lung compliance in some infants with BPD [8,9,10]. A previous study of IB use in the first 100 days of life demonstrated that in infants with BPD, IB use increases steadily in the first month of life and peaks on day of life 99 when ~10% of the population receives an IB [11]. It is not known whether IB use continues to increase after the first 100 days, though it is certainly possible given that the average length of stay for severe BPD is around 4.5 months [12]. The only randomized trial assessing albuterol use in subjects with evolving BPD patients was published more than 20 years ago and failed to demonstrate any difference in survival, incidence and severity of BPD, or duration of ventilator support or oxygen therapy [13]. More recently, the early use of bronchodilators was shown to have no significant impact on the risk of BPD or death in extremely premature infants [14]. Unfortunately, there is no information on the use of IBs from randomized studies in infants with established BPD [15]. Despite the lack of evidence for long-term benefits, IBs may be beneficial in treating acute symptoms of BPD in those infants that remain ventilator dependent [16].
The paucity of data surrounding the indications, timing of use, and effectiveness of IBs in managing symptoms of BPD, as well as concerns for adverse effects, have likely led to the wide variation in IB exposure in infants with developing or established BPD [11, 17]. For those with BPD, and especially severe BPD, the extent of IB use and the factors related to IB use are incompletely understood. This information is crucial to designing future clinical trials evaluating the safety and effectiveness of IBs. The objective of this study was to evaluate the scope of IB exposure, inter-hospital variation in IB exposure, and the clinical and demographic factors associated with IB use in BPD, and severe BPD patients using a national cohort extracted from the Pediatric Health Information System (PHIS) database.
Methods
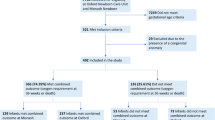
Using the PHIS database (Lenexa, KS, USA), we conducted a retrospective cohort study of infants born between January 1, 2013 and December 31, 2018. Inclusion criteria were birth prior to 32 weeks of gestation, birth weight <1500 g, admission to the NICU in the first week of life, and treatment with supplemental oxygen or respiratory support (i.e., nasal cannula (NC), high flow nasal cannula (HFNC), continuous positive airway pressure (CPAP), bi-level positive airway pressure (BiPAP), noninvasive positive pressure ventilation (NIPPV), invasive mechanical ventilation (IMV), or high frequency ventilation (HFV)) at 28 days of life. Units that did not care for at least ten infants meeting the inclusion criteria within the study period were excluded. Subjects were followed through the cessation of supplemental oxygen and respiratory support, discharge from the hospital, or the day of death, whichever came first.
Inhaled bronchodilator exposure was defined in two ways. The first was as a continuous variable, defined as the number of days an infant was exposed to any IB between day of life 28 and cessation of supplemental oxygen and respiratory support, discharge from the hospital, or the day of death, whichever came first. Secondarily, a binary variable was generated, defined as any exposure between day of life 28 and cessation of supplemental oxygen and respiratory support, discharge from the hospital, or the day of death, whichever came first. BPD was defined using modified 2018 Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop guidelines [18]. Infants were classified by the level of support needed at 36 weeks post-menstrual age (PMA). BPD categories were: (1) mild: no supplemental oxygen or respiratory support, (2) moderate: NC, and (3) severe: HFNC, CPAP, BiPAP, NIPPV, IMV, or HFV at 36 weeks PMA. Those discharged by 36 weeks PMA were considered mild BPD, while those who died prior to 36 weeks PMA were categorized as severe BPD. Further refinement of BPD based on supplemental oxygens support and x-ray finding is not possible as PHIS does not record FiO2 data or results of radiological tests performed.
Data source
The data for this study were obtained from PHIS, an administrative database containing patient billing and clinical data from members of the Children’s Hospital Association. The PHIS hospitals are 51 of the largest and most advanced children’s hospitals in America, and constitute the most demanding standards of pediatric service in America. Clinical data include patient demographics, diagnoses, level of respiratory support, and medications. Hospitals with <10 qualifying cases in the study period were eliminated, leaving 44 hospitals included in this analysis. This protocol was deemed exempt by the Institutional Review Board of the University of Nebraska Medical Center and Children’s Hospital & Medical Center.
Study variables
The PHIS database was queried to obtain daily IB administrations, daily respiratory support (NC, HFNC, CPAP, BiPAP, NIPPV, IMV, and HFV), length of stay, daily systemic corticosteroid and diuretic administrations, tracheostomy procedure (ICD-9: 31.2 or 31.29; ICD-10: 0B110F4), and demographic details of each patient. Clinical Transaction Classification (CTC) codes were used to evaluate for IB, diuretics, and corticosteroid administrations, which included: inhaled albuterol (181211.42), inhaled levalbuterol (181231.42), inhaled albuterol/ipratropium combination (181315.42), inhaled ipratropium bromide (181311.42), acetazolamide (191145), bumetanide (191131), chlorothiazide (191111), ethacrynic acid (191133), furosemide (191135), hydrochlorothiazide (191113), metolazone (191121), spironolactone (191141), dexamethasone (154035.10 or 154035.20), betamethasone (154015.10, 154015.20, 154017.20), prednisone (154083.10), prednisolone (154081.10, 154081.20), triamcinolone (154087.10, 154087.20), methylprednisolone (154071.10, 154071.20), fludrocortisone (154041.10), hydrocortisone (154063.10, 154063.20), and cortisone (154027.10). CTC codes were used to determine respiratory support needs: HFV (521161), mechanical ventilation (521166), NIPPV (521165), BiPap (521164), CPAP (521162), HFNC (521172), and oxygen delivery by cannula, tent, or mask (521171). International Classification of Disease, Ninth Revision (ICD-9) Codes were used prior to October 2015 and ICD, Tenth Revision (ICD-10) Codes thereafter to determine the diagnoses of intraventricular hemorrhage (IVH; ICD-9: 772.1, ICD-10 P77, P77.1, P77.2, P77.3, P77.9, K55.30, K55.31, K55.32, or K55.33), necrotizing enterocolitis (NEC; ICD-9: 777.5, ICD-10: P52, P52.0, P52.1, P52.2, P52.21, P52.22, P52.3, P52.4, or P52.8), and patent ductus arteriosus (PDA; ICD-9: 747.0, ICD-10: Q25.0).
Statistical analysis
Odds of IB use were compared across all levels of each covariate (gestational age, birth weight, sex, race/ethnicity, payer, average yearly census of BPD patients, level of respiratory support at 36 weeks PMA, total days of respiratory support after age 28 days, days of administration of systemic corticosteroids and diuretics after age 28 days, presence of a co-morbid condition, small for gestational age status, and presence of a tracheostomy). The proportion of patients given an IB was compared across PMA stratified by severity level using linear regression. The proportion of patients given an IB after 28 days of life and the proportion of patient-days (post 28 days of life) that IB use occurred was compared for each hospital using linear regression to determine if there was a correlation between the two. All analyses were conducted using R version 3.5.1 (R Core Team, Vienna, Austria).
Results
A total of 4986 infants from 44 hospitals required supplemental oxygen or respiratory support at 28 days of life, and 25% (1224/4986) received at least one dose of an IB between age 28 days and discharge or death. For those with severe BPD at 36 weeks PMA, 48% (664/1390) received an IB after 28 days of age. Increased IB exposure was associated with lower gestational age, lower birth weight, need for respiratory support at 36 weeks PMA, longer duration of respiratory support, need for tracheostomy, longer systemic steroid exposure, more days receiving a diuretic, male gender, and diagnosis with a major co-morbidity of IVH, NEC, or PDA (Tables 1 and 2).
The proportion of those who received an IB at each PMA is shown in Fig. 1. Up until 36 weeks PMA, <5% of infants with BPD received an IB. After this time, the proportion with mild-to-moderate BPD receiving an IB increased steadily until 60 weeks PMA, where the proportion receiving IB reaches 35–50%. The prevalence of IB use remains relatively unchanged in this range through 92 weeks PMA or 1 year corrected age for those infants still in the hospital (Fig. 1). Among infants with severe BPD, IB use rises from 25 weeks to 56 weeks PMA, reaching 35–45% of subjects receiving IBs at that time (Fig. 1). Those with severe and those with mild or moderate BPD did not differ in their rate of BPD use at baseline (p for severity level = 0.92) or across PMA (p for interaction = 0.18). Because subjects were followed through the cessation of supplemental oxygen and respiratory support, discharge to home, or the day of death, whichever came first, the number of infants studied at each gestational age changed over time peaking at 3286 at 36 weeks PMA for the mild or moderate group and decreasing thereafter as also shown in Fig. 1. While most infants were discharged home from the NICU, 276 (6%) were transferred to the pediatric ICU and remained in the study until one of the above endpoints was met.
Gray circles represent mild or moderate BPD infants and black circles represent infants with severe BPD. The denominators for the scatter plots are all the infants with the respective BPD severity who remain alive and in the hospital and so change over time. They are represented by the gray (mild or moderate BPD) and black (severe BPD) lines.
Patterns and frequency of IB use were determined for four IBs. Within this cohort of BPD patients, 1147 (23%) received albuterol, 191 (3.8%) received levalbuterol, 169 (3.4%) received ipratropium bromide, and 16 (0.3%) received albuterol/ipratropium combination. Of the BPD patients classified as severe, 621 (45%) received albuterol, 132 (9.5%) received levalbuterol, 128 (9.2%) received ipratropium bromide, and 12 (0.9%) received albuterol/ipratropium combination. Albuterol was the IB most widely prescribed IB at any point during hospitalization, as well as the IB prescribed most per patient-days among all patients (26,454 (4.7%) patient-days) and among those classified as severe (18,501 (8.8%) patient-days).
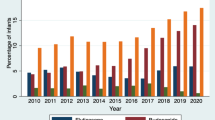
All but two hospitals in this cohort prescribed an IB to at least some of their patients with BPD. The proportion of BPD infants who received IBs after 28 days of life varied considerably by hospital, ranging from 0 to 59% of patients across 44 hospitals (Fig. 2). When IBs are examined individually, inter-hospital variability remains. Albuterol use ranged from 0 to 53% in all BPD infants, whereas levalbuterol was not used at all in 18 hospitals but was as high as 43% of BPD infants in one hospital. Ipratropium bromide use varied from 0 to 19%. Experience with BPD patients, measured by the average number of BPD patients seen by the hospital each year, was not associated with IB use and thus could not account for the variation in practice (Table 2).
To better understand IB use, the association between the number of patients receiving IBs and the length of therapy was explored by plotting the proportion of patient-days with IB exposure by the proportion of BPD patients who were ever exposed to an IB (Fig. 3). Hospitals that prescribe IBs more frequently in BPD patients also tend to prescribe them for longer, with more patient-days of IB use (r2 = 0.43, p value ≤ 0.001).
Discussion
This large multicenter retrospective study demonstrates that the use of IB in NICU patients with BPD is widespread, with 25% of the cohort receiving an IB. The most important factors associated with IB use in infants with BPD were those undergoing tracheostomy, duration of corticosteroid and diuretic treatment, increased number of days on respiratory support, and need for invasive ventilation; all of which are markers of more severe respiratory disease. We also found considerable variability in IB use between hospitals. While some hospitals used the medicines infrequently, in one hospital the majority of infants with BPD received an IB. No identifiable site characteristic was found that could explain the inter-hospital variation; however, those hospitals that used IB in a higher percentage of patients also tended to use the medications for a longer duration.
Compared with our findings of 48% of severe BPD patients receiving an IB, Bamat et al., using the PHIS database to study cumulative medicine exposures in severe BPD patients between 2007 and 2016, found that 38% of infants with severe BPD were exposed to albuterol [17]. Similar to our results, another study demonstrated significant variability in IB use, showing that the point prevalence of bronchodilator use in severe BPD patients in eight NICUs was 32% and ranged from 0 to 67% [19]. In a cohort of infants born between 230/7 and 286/7 weeks of gestation, 32% received an IB with no statistical difference between infants with BPD and without BPD at 36 weeks PMA [20]. Post-discharge use of IBs in infants who required respiratory support at 36 weeks PMA continued to increase over the first year of life, from 13% at 3 months corrected age to 31% at 12 months corrected age [21]. Albuterol was the most common IB used post-discharge, and IB use was associated with lower gestational age and a diagnosis of BPD at 36 weeks PMA.
Slaughter et al. [11] recently used the PHIS database to identify a cohort of patients with evolving BPD and determined that 33% of infants with BPD at 28 days of life received at least one dose of an IB and that usage steadily increased in the first month of life. They also described a large inter-hospital variation in bronchodilator use in their population. Similar to our findings, increased duration of positive pressure respiratory support via CPAP, NIMV, or mechanical ventilation, birth weight between 500 and 749 g, and birth at 24–26 weeks gestation were identified as factors associated with higher IB administration. Unlike their cohort, a birth weight of <500 g and birth at <24 weeks of gestation were also identified as factors related to IB use in our cohort of patients with established BPD. They did not observe a difference in IB exposure in infants with IVH, PDA, or NEC, though for all three co-morbidities we saw an increase in the odds of receiving IBs. In addition, they limited their report on bronchodilator use to the first 100 days of life and did not report data related to gestational age. For an infant born at 24 weeks, he or she has not reached their due date by 100 days of life. Thus, while their peak use of bronchodilators occurred on day 99 at 10.3%, by following BPD infants during their entire NICU course or until 1 year after their due date, we found a higher proportion of hospitalized BPD infants received IBs in the later PMA period. Lastly, a higher percentage of BPD infants received IB in their cohort compared with ours (33% vs. 25%), which may reflect changing user patterns.
We also found that higher rates of IB administration in a NICU were correlated with increased duration of IB treatment. This finding reflects the wide variability in the use of these medications, likely due to practitioner or hospital preference. Like initiating IB, there are no objective standards describing when to cease bronchodilator therapy, which likely contributes to the variation in patient-days. For practitioners who initiate IBs because they believe IBs are beneficial it may be unsurprising that they also tend to use them for a longer duration.
Knowing that a quarter of BPD infants and nearly half of severe BDP infants receive an IB, there is an urgent need to better understand the safety and efficacy of this medication in this population. It is unclear if the increased use of IB in infants with severe BPD was due to a positive clinical response, a lack of other effective therapies combined with worsening clinical status, or a result of a change in the makeup of the medical team caring for the infant. Pulmonologists, who are more familiar with IBs, may be more likely to be consulted in severe cases of BPD and recommend these drugs. Concerns related to use of albuterol include worsening bronchomalacia, tachycardia, hypertension, cardiac arrhythmias, hypokalemia, hyperglycemia, increased risk of GERD, tremor, and increased agitation [22,23,24]. Tolerance also occurs [25, 26] and evidence shows only a portion of BPD infants even respond to albuterol with improvement in their pulmonary mechanics [10]. The paucity of data surrounding the use of IB in infants with BPD, and especially those with severe BPD, is concerning given the common use of these drugs in these populations. Unfortunately, this is a pattern common in severe BPD patients as they are exposed to many medications whose risks and benefits are incompletely understood [17].
Several significant questions remain unanswered in the current literature regarding the use of IBs in infants with BPD. Even the definition of BPD itself continues to evolve with little evidence on how the diagnostic criteria impact future outcomes [27, 28]. A new definition of BPD that is based on subsequent childhood mortality rather than clinical status at 36 weeks PMA has been proposed, but remains to be thoroughly validated [29]. Another limitation of the current BPD definition is that it is static and relies on a single point in time. It is possible the use of the medications may change the respiratory support needs in a gradual manner, so studying the effects of medication use on growth trajectory and continued respiratory support after 36 weeks PMA will also be important. In addition, future studies should consider assessing the effects of the use of one medication on the need for other medications. For example, in our population higher IB use was associated with more days receiving postnatal systemic corticosteroids and diuretics but no causation can be established with our data. If earlier IB use had the potential to decreases the use or amount of systemic corticosteroids administered, however, it may be advantageous to use more IBs given the comparative adverse effects between the two groups of drugs.
Our findings must be placed in the context of several limitations. PHIS maintains quality standards for inclusion but was initially designed for administrative purposes rather than research. It collects information from medical record billing data which is potentially susceptible to error. To record data on NEC, PDA, IVH, and tracheostomy, ICD-9 and ICD-10 codes were used which can be non-specific. Hospital charge data was used to collect information about respiratory support and medication use. The PHIS database does not log FiO2 data, NC flow rates, oxyhemoglobin saturations, or radiological interpretations which are used to define BPD according to the 2018 Eunice Kennedy Shriver National Institute of Child Health and Human Development workshop guidelines [18]. Including only infants on supplemental oxygen or respiratory support at 28–30 days of life may underestimate BPD but is practical to use in this study as the medications of interest are often initiated prior to 36 weeks PMA. By only including patients who required respiratory support for three consecutive days, those with transient respiratory support needs were excluded. Although a reasonable assumption, categorizing eligible infants who were discharged home by 36 weeks PMA as mild BPD and those who died as severe BPD may result in misclassification bias. The clinical indications for use and response are not recorded in the PHIS database. A complete understanding of the decisions made by the physician is beyond the scope of this study. Causal effects of IB exposure and clinically useful outcomes such as change in BPD symptoms or severity, number of ventilator days, need for tracheostomy, need for systemic steroids, and duration of hospitalization cannot be determined from the retrospective PHIS data.
Despite these limitations, there are a number of strengths to our study. For the first time to our knowledge, this study examines the use of IB in BPD and severe BPD patients by PMA, following them during the entire NICU course up until 1 year after their estimated date of delivery. In addition, the study population comes from PHIS which comprises a large national representation of children’s hospitals and our findings are likely generalizable to most NICUs caring for BPD patients in the United States.
In summary, exposure of BPD patients to IB is widespread in the NICU, especially in cases of severe BPD. The use of IB varies widely between hospitals, with the current study unable to define any hospital related factors that could be influencing the variability. For infants with BPD, bronchodilator administration steadily increases after 36 weeks PMA until about 60 weeks PMA, and its use is associated with the need for respiratory support at 36 weeks PMA, duration of respiratory support and corticosteroid administration, and need for tracheostomy. The high IB use and variability indicates an urgent need for future research in this vulnerable population.
References
Bhandari A, McGrath-Morrow S. Long-term pulmonary outcomes of patients with bronchopulmonary dysplasia. Semin Perinatol. 2013;37:132–7.
Jensen EA, Schmidt B. Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol. 2014;100:145–57.
Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. J Pediatr. 2005;146:798–804.
Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med. 2007;161:1082–7.
Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126:443–56.
Drummond D, Hadchouel A, Torchin H, Roze JC, Arnaud C, Bellino A, et al. Educational and health outcomes associated with bronchopulmonary dysplasia in 15-year-olds born preterm. PLoS ONE. 2019;14:e0222286.
Hsieh EM, Hornik CP, Clark RH, Laughon MM, Benjamin DK Jr., Smith PB, et al. Medication use in the neonatal intensive care unit. Am J Perinatol. 2014;31:811–21.
Wilkie RA, Bryan MH. Effect of bronchodilators on airway resistance in ventilator-dependent neonates with chronic lung disease. J Pediatr. 1987;111:278–82.
Rotschild A, Solimano A, Puterman M, Smyth J, Sharma A, Albersheim S. Increased compliance in response to salbutamol in premature infants with developing bronchopulmonary dysplasia. J Pediatr. 1989;115:984–91.
Morrow DK, Schilling D, McEvoy CT. Response to bronchodilators in very preterm infants with evolving bronchopulmonary dysplasia. Res Rep Neonatol. 2015;5:113–7.
Slaughter JL, Stenger MR, Reagan PB, Jadcherla SR. Inhaled bronchodilator use for infants with bronchopulmonary dysplasia. J Perinatol. 2015;35:61–6.
Morrow CB, McGrath-Morrow SA, Collaco JM. Predictors of length of stay for initial hospitalization in infants with bronchopulmonary dysplasia. J Perinatol. 2018;38:1258–65.
Denjean A, Paris-Llado J, Zupan V, Debillon T, Kieffer F, Magny JF, et al. Inhaled salbutamol and beclomethasone for preventing broncho-pulmonary dysplasia: a randomised double-blind study. Eur J Pediatr. 1998;157:926–31.
Koch A, Kreutzer KB, Poets C, Engel C, Bassler D, Group NET. The impact of inhaled bronchodilators on bronchopulmonary dysplasia: a nonrandomized comparison from the NEuroSIS trial. J Matern Fetal Neonatal Med. 2019: 1–3. https://doi.org/10.1080/14767058.2019.1590331.
Ng G, da Silva O, Ohlsson A. Bronchodilators for the prevention and treatment of chronic lung disease in preterm infants. Cochrane Database Syst Rev. 2016;12:CD003214.
Sosulski R, Abbasi S, Bhutani VK, Fox WW. Physiologic effects of terbutaline on pulmonary function of infants with bronchopulmonary dysplasia. Pediatr Pulmonol. 1986;2:269–73.
Bamat NA, Kirpalani H, Feudtner C, Jensen EA, Laughon MM, Zhang H, et al. Medication use in infants with severe bronchopulmonary dysplasia admitted to United States children’s hospitals. J Perinatol. 2019;39:1291–9.
Higgins RD, Jobe AH, Koso-Thomas M, Bancalari E, Viscardi RM, Hartert TV, et al. Bronchopulmonary dysplasia: executive summary of a workshop. J Pediatr. 2018;197:300–8.
Guaman MC, Gien J, Baker CD, Zhang H, Austin ED, Collaco JM. Point prevalence, clinical characteristics, and treatment variation for infants with severe bronchopulmonary dysplasia. Am J Perinatol. 2015;32:960–7.
Greenberg JM, Poindexter BB, Shaw PA, Bellamy SL, Keller RL, Moore PE, et al. Respiratory medication use in extremely premature (<29 weeks) infants during initial NICU hospitalization: results from the prematurity and respiratory outcomes program. Pediatr Pulmonol. 2020;55:360–8.
Ryan RM, Keller RL, Poindexter BB, D’Angio CT, Shaw PA, Bellamy SL, et al. Respiratory medications in infants <29 weeks during the first year postdischarge: the Prematurity and Respiratory Outcomes Program (PROP) consortium. J Pediatr. 2019;208:148–55 e143.
National Heart, Lung, and Blood Institute and the National Asthma Education and Prevention Program. Expert Panel Report 3 (EPR 3): guidelines for the diagnosis and management of asthma. 2007 [cited 2020 Jan 4] https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma.
Doull IJ, Mok Q, Tasker RC. Tracheobronchomalacia in preterm infants with chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 1997;76:F203–205.
Crowell MD, Zayat EN, Lacy BE, Schettler-Duncan A, Liu MC. The effects of an inhaled beta(2)-adrenergic agonist on lower esophageal function: a dose-response study. Chest. 2001;120:1184–9.
van der Woude HJ, Winter TH, Aalbers R. Decreased bronchodilating effect of salbutamol in relieving methacholine induced moderate to severe bronchoconstriction during high dose treatment with long acting beta2 agonists. Thorax. 2001;56:529–35.
Haney S, Hancox RJ. Rapid onset of tolerance to beta-agonist bronchodilation. Respir Med. 2005;99:566–71.
Jensen EA, Wright CJ. Bronchopulmonary dysplasia: the ongoing search for one definition to rule them all. J Pediatr. 2018;197:8–10.
Jobe AH, Bancalari EH. Controversies about the definition of bronchopulmonary dysplasia at 50 years. Acta Paediatr. 2017;106:692–3.
Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. an evidence-based approach. Am J Respir Crit Care Med. 2019;200:751–9.
Funding
RM and EK receive support from the Office of the Director of the National Institutes of Health under award UG1OD024953. The remaining authors did not receive funding.
Author information
Authors and Affiliations
Contributions
JE conceptualized and designed the study, interpreted the data, drafted the initial paper, and approved the final paper as submitted. EK conceptualized and designed the study, accessed the PHIS database, interpreted the data, and approved the final paper as submitted. CL, RM and EP assisted with study design, participated in interpretation of the data, reviewed, and revised the paper, and approved the final paper as submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest relevant to this article to disclose. Dr RM from time to time provides expert advice on legal matters.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Euteneuer, J.C., Kerns, E., Leiting, C. et al. Inhaled bronchodilator exposure in the management of bronchopulmonary dysplasia in hospitalized infants. J Perinatol 41, 53–61 (2021). https://doi.org/10.1038/s41372-020-0760-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-0760-8