Abstract
Objective
We investigated how diagnosis and injury location on neonatal brain MRI following onset of acute provoked seizures was associated with short term outcome.
Study design
A multicenter cohort of neonates with acute provoked seizures enrolled in the Neonatal Seizure Registry. MRIs were centrally evaluated by a neuroradiologist for location of injury and radiologic diagnosis. Clinical outcomes were determined by chart review. Multivariate logistic regression was used to examine the association between MRI findings and outcomes.
Results
Among 236 newborns with MRI at median age 4 days (IQR 3–8), 91% had abnormal MRI. Radiologic diagnoses of intracranial hemorrhage (OR 3.2 [1.6–6.5], p < 0.001) and hypoxic-ischemic encephalopathy (OR 2.7 [1.4–5.4], p < 0.003) were associated with high seizure burden. Radiologic signs of intracranial infection were associated with abnormal neurologic examination at discharge (OR 3.9 [1.3–11.6], p < 0.01).
Conclusion
Findings on initial MRI can help with expectant counseling on short-term outcomes following acute provoked neonatal seizures.
Similar content being viewed by others
Introduction
Neonatal acute symptomatic or acute provoked seizures, defined as seizures due to acute brain injury, affect approximately 1–3 out of every 1000 live births in the United States each year [1, 2]. Acute provoked seizures are often the first sign of neurologic dysfunction in neonates who have suffered acute brain injury. The most common causes of seizures in neonates are acute brain injury due to hypoxic-ischemic encephalopathy (HIE), ischemic stroke, or intracranial hemorrhage [3]. Children with acute provoked seizures are at high risk of adverse neurodevelopmental outcome, including post-neonatal epilepsy, developmental delay, intellectual disability, and cerebral palsy [1]. Despite advances in neonatal neurocritical care, relatively little is known about the risk factors that predispose some neonates to experience a higher seizure burden or abnormal neurologic examination at hospital discharge after acute provoked seizures.
Magnetic resonance imaging (MRI) is increasingly used to determine seizure etiology and predict long-term prognosis for neonates with seizures, but little is known about the relationship between seizures and specific neuroimaging findings in this population. Prior studies have been limited by single center enrollment [1, 4,5,6,7,8]. A detailed understanding of the relationship between early imaging findings and short-term clinical outcomes has the potential to guide parent counseling during the neonatal period.
We evaluated a large, prospective multicenter cohort of infants with acute provoked seizures enrolled in the Neonatal Seizure Registry to test the hypothesis that the etiology and location of brain injury detected on early neonatal brain MRI following onset of acute provoked seizures are associated with seizure burden and neurologic outcomes at hospital discharge.
Methods
Study design
This was an ancillary study of a prospective, multicenter cohort of infants with acute provoked neonatal seizures born between 7/2015 and 3/2018 who survived the neonatal admission, enrolled at seven National Seizure Registry (NSR) sites (NCT02789176) [9, 10]. Each site has a level IV neonatal intensive care unit (NICU) and followed the American Clinical Neurophysiology Society (ACNS) guidelines for continuous electroencephalographic monitoring (cEEG) in neonates [11]. The local institutional review board at each site approved the study, and neonates were enrolled after informed parental consent.
Inclusion and exclusion criteria
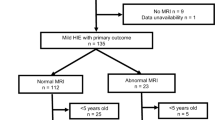
NSR enrollment was determined at each site by the study site investigator, a pediatric neurologist. Enrollment criteria were pre-established (Fig. 1): (1) neonate with EEG-confirmed seizure at either the study site or the referring hospital, or (2) neonate treated with antiseizure medication for suspected clinical seizures with a clinical history, including event semiology, supporting the diagnosis of seizures, and (3) seizures were due to an acute symptomatic cause. Neonates were excluded if events were determined not to be seizures based on history, semiology, or cEEG, or were determined not to be transient or secondary to an acute provoked cause (i.e., genetic or neonatal epilepsy syndromes). Neonates were also excluded from the present ancillary analysis if there was no available brain MRIs or if the brain MRI was of nondiagnostic quality. Only children who survived the neonatal admission were enrolled.
Measurements
Clinical and demographic data
Clinical information was obtained prospectively upon enrollment by chart review performed at enrollment sites, performed by the study team led by the site investigator, a pediatric neurologist. Clinical information included sex, preterm birth (defined as less than 37 weeks gestation at birth), and complex medical course (defined binarily by any combination of congenital heart disease, cardiac failure, extracorporeal membrane oxygenation treatment, or dialysis).
MRI
All MRI studies were performed using local institutional protocols. The first MRI after neonatal seizure onset was centrally reviewed by a board-certified, fellowship-trained pediatric neuroradiologist with 7 years of experience (YL). The first ten MRIs were also reviewed by a pediatric neuroradiologist with 40 years of experience (AJB) to establish consensus in scoring methodology.
Studies that were not of diagnostic quality, based on the degree of motion degradation, were identified and excluded. Studies were evaluated for available sequences [T1, T2, Diffusion Weighted Imaging (DWI), Susceptibility Weighted Imaging (SWI), and T1 post-contrast].
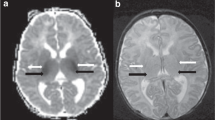
In generating the primary imaging predictors, a binary scoring system (present/absent) was used to assess for any signal abnormality corresponding to injury involving cortex and deep gray nuclei (Fig. 2). Examples of cortical injury include reduced diffusion signifying acute ischemia, T1 hyperintensity compatible with laminar necrosis, and loss of the cortical ribbon compatible with cortical edema. Examples of deep gray injury include reduced diffusion signifying acute ischemia, susceptibility artifact compatible with hemorrhage, abnormal T1 hyperintensity or T2 hypointensity signifying evolving injury in the setting of HIE, and T2 hyperintensity signifying edema.
A similar binary model (present/absent) was used to categorize MRI studies regarding specific radiologic diagnoses including: normal, HIE, focal ischemic stroke (arterial, venous, or other etiology), intracranial hemorrhage (ICH), suspected infection (based on findings of leptomeningeal enhancement, and reduced diffusion signifying abscess, empyema or ventriculitis), and “other” (including suspected hypoglycemia, chronic injury including encephalomalacia, low white matter volume, and periventricular white matter injury of prematurity) (Fig. 3). Small parturitional subdural hematoma was not scored as abnormality, as it was felt to be common and not contributory to seizure etiology. Neonates could have more than one radiologic diagnosis and could have injury in none, one, or both scored locations.
A Axial ADC map demonstrating arterial ischemic stroke in the left middle cerebral artery territory (white arrow). B Axial ADC map demonstrating reduced diffusion in the deep gray nuclei and subcortical white matter, compatible with hypoxic ischemic injury. C Axial T2 weighted imaging demonstrating large subpial hemorrhage in the medial right frontal lobe (asterisk) with associated hemorrhagic injury of the right frontal lobe. D Axial T1-weighted post-contrast imaging demonstrating leptomeningeal enhancement (arrowheads) in the setting of neonatal meningitis/intracranial infection.
Clinical outcomes data
The primary clinical seizure etiology was determined by the enrolling site investigator based on a combination of clinical and radiologic factors and categorized as follows: HIE, ischemic stroke, ICH, infection, hypoglycemia, and other.
Two primary clinical outcomes of interest were collected by the site investigator for the purposes of this analysis. First, EEG-seizure burden was assessed on a 5-point scale[3]: None, rare (<7), many isolated (> = 7), frequent recurrent EEG seizures, status epilepticus [12], or documentation inadequate to quantify. For analysis, these categories were dichotomized into low seizure burden (defined as rare seizures), and high seizure burden (defined as many isolated, frequent recurrent EEG seizures, or status epilepticus) [10]. Second, neurologic examination at time of discharge was determined by the site investigator as normal or abnormal (defined as any abnormality in consciousness, tone, or reflexes).
Two additional secondary outcomes of interest were evaluated: (1) presence of EEG-only seizures, defined as EEG seizures without recognized clinical correlate, and (2) incomplete response to loading dose of antiseizure medication, defined as ongoing seizures after 30 min despite a loading dose of antiseizure medication (defined as at least 20 mg/kg for phenobarbital and phenytoin/fosphenytoin and 40 mg/kg for levetiracetam).
Statistical analysis
In multiple logistic regression, imaging predictors including location of injury and radiologic diagnosis were modeled in association with the outcomes of seizure burden and abnormal discharge examination, while controlling for clinically identified potential confounders of sex, preterm birth, and complex medical course. In multivariate analysis, predictors reaching statistical significance of p < 0.1 were modeled in association with outcomes using logistic regression, also controlling for clinically identified potential confounders of sex, preterm birth, and complex medical course. We used backward elimination of variables with p < 0.05 to arrive at our final, multivariate reported model.
Analyses and adjustments were conducted using Stata version 16 (College Station, TX) [13].
Results
Demographics and MRI diagnoses
Two hundred fifty-two newborns were enrolled. Sixteen newborns were excluded from the current analysis due to incomplete imaging data. Table 1 provides the demographic information for the 236 infants included in this analysis.
MRI was performed at a median age 4 (IQR 3 to 8) days after birth. No studies were nondiagnostic in quality. MRIs contained the following sequences: T2 99%, DWI 97%, T1 94%, SWI 89%, post-contrast T1 26%.
Cortical injury was present in 118/236 (50%) neonates, and deep gray injury was present in 89/236 (38%).
Radiologic etiologies included focal ischemic stroke in 85 (36%), HIE in 67 (28%), ICH in 22 (9%), suspected infection in 16 (7%), and other etiologies in 30 (12%). Twenty-two (9%) had a normal MRI. More than one radiologic diagnosis was present in 42 (18%). Fourteen of 22 (64%) neonates with a normal MRI had a clinical diagnosis of HIE as the underlying seizure etiology. Among these 14 neonates with normal MRI and HIE, 13 (93%) had undergone therapeutic hypothermia. Supplementary Table 1 provides the distribution of radiologic diagnoses, in comparison to primary clinical diagnoses.
MRI findings and primary outcomes
Seizure burden
Neonates with a normal MRI were less likely to have a high seizure burden (3/22, 14%) compared to those with an abnormal MRI (127/214, 59%, OR 0.13 [0.04–0.45], p < 0.001, Table 2). A high seizure burden was more likely among children with a radiologic diagnosis of HIE (67% with HIE vs 50% without HIE, OR 2.4 [1.3–4.4], p < 0.006) and less likely among those with a radiologic diagnosis of “other” (39% with “other” diagnoses vs 59% with HIE, ischemic stroke, intracranial hemorrhage, or intracranial infection, OR 0.44 [0.22–0.85], p < 0.02). Neonates with ICH had a trend toward high seizure burden (68% with ICH vs 51% without ICH, OR 1.8 [0.96–3.4], p < 0.07). Cortical injury was significantly associated with a high seizure burden (68% with cortical injury vs 42% without cortical injury, OR 3.5 [1.9–6.4], p < 0.001). Deep gray injury was also associated with a high seizure burden (63% with deep gray injury vs 50% without deep gray injury, OR 1.7 [1.0–3.0], p = 0.045).
In the multivariate model, a diagnosis of HIE (OR 2.7 [1.4–5.4], p < 0.003), ICH (OR 3.2 [1.6–6.5], p < 0.001), or cortical injury (OR 3.5 [1.9–6.4], p < 0.001) remained independently associated with high seizure burden.
Neurologic examination at discharge
Neonates with MRI evidence of HIE were more likely to have an abnormal neurologic examination at discharge (42% with HIE vs 27% without HIE, OR 2.2 [1.1–3.7], p < 0.02). Neonates with a radiologic diagnosis of infection were also more likely to have an abnormal neurologic examination at discharge (63% with infection vs 29% without infection, OR 4.2 [1.4–12.0], p < 0.008). Deep gray injury on early MRI was also associated with abnormal discharge examination (45% with deep gray injury vs 22% without deep gray injury, OR 2.8 [1.6–5.0], p < 0.001).
In the multivariate model, a radiologic diagnosis of infection (OR 3.9 [1.3–11.6], p < 0.01) and deep gray injury (OR 2.7 (1.6–5.0], p < 0.001) remained independently associated with abnormal neurologic examination at discharge.
MRI findings and secondary outcomes
EEG-only seizures
Neonates with ischemic stroke were less likely to have EEG-only seizures (11% with stroke vs 23% without stroke, OR 0.42 [0.5–0.9], p < 0.03, Supplementary Table 2). Cortical injury was also negatively associated with EEG-only seizures (7% with cortical injury vs 30% without cortical injury, OR 0.2 [0.09–0.45], p < 0.0001).
In the multivariate model, the negative association between cortical injury and EEG-only seizures remained statistically significant (OR 0.2 [0.09–0.45], p < 0.0001).
Treatment resistant seizures
Neonates with a normal MRI were less likely to have treatment resistant seizures (22% with normal MRI compared to 67% with abnormal MRI, OR 0.13 [0.05–0.38], p < 0.0001). Among the radiologic diagnoses, HIE was significantly associated with treatment resistant seizures (84% with HIE vs 55% without HIE, OR 5.2 [2.4–11.5], p < 0.0001). Cortical injury was significantly associated with treatment resistant seizures (73% with cortical injury vs 53% without cortical injury, OR 2.6 [1.5–4.7], p < 0.001).
In the multivariate model, a diagnosis of HIE (OR 4.9 [2–10.8], p < 0.0001) and cortical injury (OR 4.9 [2.2–10.8], p < 0.001) remained associated with treatment resistant seizures.
Discussion
In this large, prospective, multi-center cohort of neonates with acute provoked seizures and MRI at a median of 4 days, specific imaging abnormalities were associated with short-term clinical outcomes and seizure characteristics. These findings can be used to guide parental counseling in the neonatal period. Ninety one percent of neonates with acute provoked seizures had an abnormal brain MRI. In the multivariate analysis, HIE and ICH were associated with high seizure burden, and HIE was associated with treatment resistant seizures. MRI evidence of intracranial infection was associated with an abnormal neurologic examination at discharge. With regards to location of injury, cortical injury was associated with higher seizure burden and treatment resistant seizures, while deep gray injury was associated with higher odds of an abnormal neurologic examination at discharge. These imaging-focused data add further detail to the existing knowledge about neonates with acute provoked seizures [3], allowing for more detailed counseling on short-term outcomes based on specific imaging diagnoses and locations of injury.
In multivariate analysis, ICH was associated with highest odds of high seizure burden. Prior studies have found that acute symptomatic seizures may be associated with any form of intracranial hemorrhage, whether intra- or extra-axial, although parturitional subdural hemorrhage, which was not scored in this study, is not typically associated with seizures [14]. In one study, seizure rates did not differ based on traumatic versus non-traumatic cause of intracranial hemorrhage, but parenchymal injury was associated with higher odds of acute symptomatic seizures [15]. Intraventricular hemorrhage is thought to cause seizures when associated with parenchymal injury, such as from periventricular hemorrhagic infarction, and is seen more commonly in preterm neonates [14, 16].
Prior studies have found that HIE to be the most common cause of acute provoked seizures [3], and although the incidence has decreased with therapeutic hypothermia, approximately half of children with HIE have seizures in spite of cooling [16]. In our entire cohort of infants with acute provoked seizures, 30% underwent therapeutic hypothermia, and amongst those with a radiologic diagnosis of HIE, 39% underwent hypothermia. This seemingly low rate of hypothermia can be explained by the fact that infants were enrolled into our study on the basis of EEG-confirmed seizures during the neonatal admission, not based on Sarnat [17] criteria for HIE and therapeutic hypothermia, and many of these seizures likely occurred beyond the 6 h window for therapeutic hypothermia initiation. Many of these infants with HIE as the underlying seizure etiology also had complex medical conditions that precluded them from meeting criteria for therapeutic hypothermia.
Unlike prior studies, in our study, focal ischemic stroke was also the most common radiologic etiology for acute provoked seizures. The total number of infants with HIE by radiologic diagnosis was lower than that by clinical diagnosis (28% vs 42%) and the total number of ischemic strokes by radiologic diagnosis was higher compared to clinical diagnosis (36% vs 26%). This is likely due to radiologic interpretation of focal areas of reduced diffusion as perinatal stroke, which in the clinical context of peripartum asphyxia, would clinically be assigned as sequela of HIE. One prior study suggested that up to 5% of children with neonatal encephalopathy presumed to be secondary to HIE actually had ischemic stroke on MRI, and thus the imaging diagnosis may be a more accurate reflection of the true etiology [18]. Prior research from our group on acute provoked seizures in neonates did not find a difference in seizure burden based on clinical etiology for seizures after adjusting for potential confounders [3], thus the differences in seizure burden based on radiologic etiology adds to existing knowledge and may inform counseling during the immediate hospitalization period.
Neonates with MRI evidence of intracranial infection, such as leptomeningeal enhancement or purulent reduced diffusion, had higher odds of abnormal neurologic examination at discharge compared to those without MRI evidence of infection. Neonatal bacterial or viral meningitis is known to cause significant morbidity, often leading to subsequent complications including stroke, vasculopathy, venous thrombosis, hydrocephalus, and/or cerebral abscess formation. Acute seizures in the setting of infection have been known to persist longer than other etiologies, possibly related to ongoing inflammation during treatment [14]. The poor short-term outcomes in this population may reflect the additional complications that arise secondarily as a result of infection.
Injury to the cortex, regardless of underlying etiology, was significantly associated with higher seizure burden and treatment refractory seizures. The cortex, which contains principal and interneurons that form networks of synaptic circuits throughout the brain, is subject to excitability, which is dysregulated in the setting of seizures [19]. Neonates with cortical injury also demonstrated lower odds of EEG-only seizures.
Deep gray injury was associated with an abnormal neurologic examination at discharge. These findings are consistent with existing neonatal HIE literature, as injury to these structures is strongly associated with adverse motor, cognitive and language outcomes in this population [20, 21]. Prior research from our group has shown that deep gray injury in neonates with acute symptomatic seizures was also significantly associated with subsequent development of infantile spasms later in life [22]. Thus, the identification of deep gray injury in this population has implications for not only for counseling on short term outcomes, but ramifications for long term care planning and early intervention.
Although we report imaging findings from a large cohort of neonates with acute provoked seizures enrolled prospectively from seven quaternary pediatric centers, our study has limitations. First, although the clinical MRIs were centrally reviewed by a pediatric neuroradiologist with expertise in neonatal imaging, they were acquired using heterogeneous imaging protocols that varied across institutions. As these protocols varied in available sequences and sequence parameters, it is possible that some abnormalities were variably detected based on available imaging. Second, we report only short-term outcomes; detailed evaluation of the association between MRI findings and childhood neurodevelopment in infants with acute provoked seizures is planned. Third, as neonates included in this study were initially enrolled as part of a larger study on long-term neurodevelopmental outcomes after acute symptomatic seizures, neonates who did not survive the neonatal admission were excluded, and the results of our study are not applicable in the setting of death prior to discharge from the neonatal hospitalization. Fourth, we did not investigate acuity of imaging injury, which is important to establish the timing of injury and highlights the importance of the maternal-placental-fetal triad and trimester-specific gene-environment factors in the development of neonatal central nervous system disease, including neonatal seizures [23]. Investigating additional gene-environment interactions and the maternal-placental-fetal triad will be an important aspect of future work in this field.
Conclusion
In the context of neonatal acute provoked seizures, MRI findings of ICH or HIE as the underlying etiology for acute provoked seizures, and/or cortical location of injury were associated with high seizure burden. Radiologic evidence of infection and/or injury to the deep gray nuclei were associated with an abnormal neurologic examination at discharge. These findings on initial MRI can aid in parental counseling on short term outcomes for neonates with acute provoked seizures. Future work should characterize the association between brain MRI findings and neurodevelopmental outcomes after acute provoked neonatal seizures.
Data availability
Multi-institutional data was collected as part of the Neonatal Seizure Registry and may be available upon request by contacting the corresponding author.
References
Glass HC, Grinspan ZM, Shellhaas RA. Outcomes after acute symptomatic seizures in neonates. Semin Fetal Neonatal Med. 2018;23:218–22.
Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr. 2009;154:24–8.
Glass HC, Shellhaas RA, Wusthoff CJ, Chang T, Abend NS, Chu CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103.e1.
Glass HC, Hong KJ, Rogers EE, Jeremy RJ, Bonifacio SL, Sullivan JE, et al. Risk factors for epilepsy in children with neonatal encephalopathy. Pediatr Res. 2011;70:535–40.
McDonough TL, Paolicchi JM, Heier LA, Das N, Engel M, Perlman JM, et al. Prediction of future epilepsy in neonates with hypoxic-ischemic encephalopathy who received selective head cooling. J Child Neurol. 2017;32:630–7.
López-Espejo M, Hernández-Chávez M, Huete I. Clinical and radiological risk factors for poststroke epilepsy in childhood. Epilepsy Behav. 2018;88:113–6.
Jung DE, Ritacco DG, Nordli DR, Koh S, Venkatesan C. Early anatomical injury patterns predict epilepsy in head cooled neonates with hypoxic-ischemic encephalopathy. Pediatr Neurol. 2015;53:135–40.
Gano D, Sargent MA, Miller SP, Connolly MB, Wong P, Glass HC, et al. MRI findings in infants with infantile spasms after neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol. 2013;49:401–5.
Glass HC, Soul JS, Chu CJ, Massey SL, Wusthoff CJ, Chang T, et al. Response to antiseizure medications in neonates with acute symptomatic seizures. Epilepsia. 2019;60:e20–4.
Glass HC, Soul JS, Chang T, Wusthoff CJ, Chu CJ, Massey SL, et al. Safety of early discontinuation of antiseizure medication after acute symptomatic neonatal seizures. JAMA Neurol. 2021;94143:1–9.
Shellhaas RA, Chang T, Tsuchida T, Scher MS, Riviello JJ, Abend NS, et al. The American clinical neurophysiology society’s guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–7.
Tsuchida TN, Wusthoff CJ, Shellhaas RA, Abend NS, Hahn CD, Sullivan JE, et al. American Clinical Neurophysiology Society Standardized EEG Terminology and Categorization for the Description of Continuous EEG Monitoring in Neonates: Report of the American Clinical Neurophysiology Society Critical Care Monitoring Committee. J Clin Neurophysiol. 2013;30. https://journals.lww.com/clinicalneurophys/Fulltext/2013/04000/American_Clinical_Neurophysiology_Society.9.aspx.
StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP.
Soul JS. Acute symptomatic seizures in term neonates: etiologies and treatments. Semin Fetal Neonatal Med. 2018;23:183–90.
Bansal S, Kebede T, Dean NP, Carpenter JL. Predictors of acute symptomatic seizures after intracranial hemorrhage in infants*. Pediatr Crit Care Med. 2014;15:750–5.
Glass HC, Shellhaas RA. Acute symptomatic seizures in neonates. Semin Pediatr Neurol. 2019;32:100768.
Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arch Neurol. 1976;33:696–705.
Ramaswamy V, Miller SP, Barkovich AJ, Partridge JC, Ferriero DM. Perinatal stroke in term infants with neonatal encephalopathy. Neurology. 2004;62:2088–91.
Chapter 1, Basic Mechanisms Underlying Seizures and Epilepsy. In: Bromfield EB, Cavazos JE, Sirven JI, editors. An introduction to epilepsy. West Hartford (CT): American Epilepsy Society; 2006.
Martinez-Biarge M, Diez-Sebastian J, Kapellou O, Gindner D, Allsop JM, Rutherford MA, et al. Predicting motor outcome and death in term hypoxic-ischemic encephalopathy. Neurology. 2011;76:2055–61.
Tharmapoopathy P, Chisholm P, Barlas A, Varsami M, Gupta N, Ekitzidou G, et al. In clinical practice, cerebral MRI in newborns is highly predictive of neurodevelopmental outcome after therapeutic hypothermia. Eur J Paediatr Neurol. 2020;25:127–33.
Glass HC, Grinspan ZM, Li Y, McNamara NA, Chang T, Chu CJ, et al. Risk for infantile spasms after acute symptomatic neonatal seizures. Epilepsia. 2020;61:2774–84.
Scher MS. “The first thousand days” define a fetal/neonatal neurology program. Front Pediatr 2021;9. https://doi.org/10.3389/fped.2021.683138.
Funding
YL was funded by the Radiological Society of North America Research Scholar Award 2019-2021 through grant RSCH1917.
Author information
Authors and Affiliations
Contributions
YL: substantial contributions to conception and design, data analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. AS: substantial contributions to conception and design, analysis and interpretation of data; revising article critically for important intellectual content; and final approval of the version to be published. AJB: substantial contributions to conception and design, revising article critically for important intellectual content; and final approval of the version to be published. TC: acquisition of data; revising article critically for important intellectual content. CJC: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. SLM: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. NSA: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. MEL: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. CT: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. AN: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. LSF: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. ER: acquisition of data; revising article critically for important intellectual content, and final approval of the version to be published. AC: analysis and interpretation of data; revising article critically for important intellectual content, and final approval of the version to be published. CEM: analysis and interpretation of data; revising article critically for important intellectual content, and final approval of the version to be published. RAS: substantial contributions to conception and design, data analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published. HCG: substantial contributions to conception and design, data analysis and interpretation of data; drafting the article or revising it critically for important intellectual content; and final approval of the version to be published.
Corresponding author
Ethics declarations
Competing interests
RS is the only author with relevant disclosures, including: Associate Editor for Neurology; consultant for the Epilepsy Study Consortium; and receives royalties from UpToDate for authorship of topics related to neonatal seizures. The other authors have no relevant disclosures or conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Scheffler, A., Barkovich, A.J. et al. Neonatal brain MRI and short-term outcomes after acute provoked seizures. J Perinatol 43, 1392–1397 (2023). https://doi.org/10.1038/s41372-023-01723-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-023-01723-3