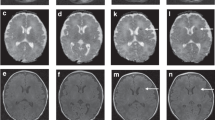
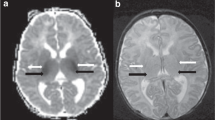
Abstract
Background
Hypothermia is widely used for infants with hypoxic–ischemic neonatal encephalopathy but its impact remains poorly described at a population level. We aimed to describe brain imaging in infants born at ≥36 weeks’ gestation, with moderate/severe encephalopathy treated with hypothermia.
Methods
Descriptive analysis of brain MRI and discharge neurological examination for infants included in the French national multicentric prospective observational cohort LyTONEPAL.
Results
Among 575 eligible infants, 479 (83.3%) with MRI before 12 days of life were included. MRI was normal for 48.2% (95% CI 43.7–52.8). Among infants with brain injuries, 62.5% (95% CI 56.2–68.5) had damage to more than one structure, 19.8% (95% CI 15.0–25.3) showed a pattern-associating injuries of basal ganglia/thalami (BGT), white matter (WM) and cortex. Overall, 68.4% (95% CI 62.0–74.3) of infants with normal MRI survived with a normal neurological examination. The rate of death was 15.4% (95% CI 12.3–19.0), predominantly for infants with the combined BGT, cortex, and/or WM injuries.
Conclusions
Among infants with neonatal encephalopathy treated with hypothermia, two-thirds of those with normal MRI survived with a normal neurological examination at discharge. When present, brain injuries often involved more than one structure.
Trial registration
The trial was registered at ClinicalTrials.gov (NCT02676063).
Impact
-
In this multicentric cohort of infants with neonatal encephalopathy (LYTONEPAL) two-thirds survived with normal MRI and neurological examination at discharge.
-
In total, 10% of newborns showed a pattern associating injuries of the basal ganglia—thalami, white matter, and cortex, which was correlated with a high risk of death at discharge.
-
The evolution of MRI techniques and sequences in the era of hypothermia calls for a revisiting of imaging protocol in neonatal encephalopathy, especially for the timing.
-
The neurological examination did not give evidence of brain injuries, thus questioning the reproducibility of the clinical exam or the neonatal brain functionality.
Similar content being viewed by others
Introduction
In high-income countries, neonatal encephalopathy (NE) related to intrapartum hypoxic–ischemic events or hypoxic–ischemic encephalopathy (HIE), occurs in 0.86–1.54‰ of live term births.1,2 Perinatal brain hypoxia and ischemia induce a complex biochemical cascade leading to neuronal cell death and significant brain injury.
Magnetic resonance imaging (MRI) is increasingly applied to quantify the degree of brain injury and to identify the specific brain regions involved, providing both diagnostic and prognostic information.3,4,5,6 The timing of the MRI may affect the identification of injuries.3,4 For MRI performed early, before 7 days of life, diffusion-weighted imaging (DWI) is an essential sequence. When MRI is performed after the first week of life, DWI should be analyzed with caution because of possible pseudonormalization.7,8,9
Four predominant MRI injury locations have been observed:5,9,10,11 basal ganglia and thalami (BGT), posterior-limb internal capsule (PLIC), cortex, and white matter (WM).3,4,5,6 BGT injuries are the most severe, known to be associated with poor motor and cognitive outcome.5,10,12,13,14,15 There is now evidence that therapeutic hypothermia (TH) for infants who may have experienced lack of oxygen at birth reduces death or disability, without increasing disability in survivors.16 Thus, recommendations are to provide TH to infants with severe to moderate NE related to HIE within 6 h (hr) after birth.16,17
Cohort studies, providing data for infants with NE treated with TH, may lead to a better understanding of the impact of TH on brain injuries. The cohort “Long-Term Outcome of Neonatal hypoxic EncePhALopathy in the era of neuroprotective treatment with hypothermia” (LyTONEPAL) is a national cohort study of term and late preterm infants with NE, conducted in France between 2015 and 2017.18 The objectives of this present study were to detail the practices of brain MRI (timing, sequences) in a cohort of infants with NE treated by therapeutic hypothermia and to report the incidence and characteristics of observed brain injuries. The secondary objective was to report the associations between MRI findings and short-term outcomes, including death or survival with normal/abnormal neurological examination.
Methods
Population
Neonates included in the LyTONEPAL cohort were born at ≥34 weeks’ gestational age between September 2015 and March 2017, and diagnosed with HIE. Sixty-eight French centers participated in the LyTONEPAL study. Criteria for HIE included (1) early neurological distress with clinical signs of moderate to severe encephalopathy on a standardized neurological examination performed by a senior investigator; (2) biological criteria of asphyxia during the first hour of life, including (a) severe biological signs of asphyxia (pH ≤ 7.0 or base deficit ≥16 mmol/l or lactate level ≥11 mmol/l) or (b) moderate signs of asphyxia (pH 7.01–7.15 or base deficit 10–15.9 mmol/l, or lactate level 8–11 mmol/l) or no available biological signs of asphyxia, associated with an acute perinatal event (cord prolapse, head retention, retro-placental hematoma, uterine rupture, cord rupture, amniotic embolism, fetal–maternal hemorrhage, or maternal hemodynamic shock).
For the estimation of the neurological status at inclusion, neonatologists were provided with a list of items for the neurological examination: neonatal level of vigilance, tone, reactivity, archaic reflexes, and clinical seizures or mydriasis. Moderate HIE was defined by lethargy, hyper-reflexia, myosis, bradycardia, seizures, hypotonia with a weak suck and poor Moro reflex; and severe HIE by stupor, flaccidity, small to mid-position pupils that react poorly to light, decreased stretch reflexes, hypothermia or absent Moro reflex.18
Neonates with congenital malformations, chromosomal disorders, and congenital neuromuscular disorders were not included or were subsequently excluded. Details about the cohort are available elsewhere.18 In the current study, the analysis was restricted to newborns born at ≥ 36 weeks’ gestation, receiving TH, with at least one MRI scan performed between day (D) 0 and D12 of life. MRI scans performed after the second week of life were not included.
Data collection
Perinatal factors
Maternal characteristics included mode of delivery (cesarean, instrumental, or non-instrumental vaginal delivery), outborn birth (birth outside a center with TH access), and acute perinatal event, and the presence of fetal heart abnormalities.
Patient characteristics included gestational age, birth weight, sex, Apgar score at 5 and 10 min, intubation in the delivery room, moderate/severe NE according to Sarnat grade (grade II and III),19 biological parameters such as pH and lactate level at birth, glycemia at admission, hypoglycemia during the first 24 h (defined as glucose level < 2.2 mmol/l), C-reactive protein level during the first 72 h of life, time to reach 34 °C with TH (hours), and seizures (clinical or electroencephalographic) during hospitalization.
MRI
MRI data were prospectively collected with a common reporting template developed by an expert committee of pediatric radiologists in France (Supplementary data 1) and were all validated by a senior pediatric radiologist for each center. No central MRI reading was done. Data used for this study was taken from these clinical reports and included the timing of MRI acquisition, type of sequence (at least DWI, T1, and T2 weighted imaging), quality of scans (“good or excellent” with no artifact on the three axial DWI, T1, and T2 sequences or poor/uninterpretable with artifacts present on at least one of the three sequences), presence of injuries according to seven brain regions of interest (BGT, WM, cortex, PLIC, corpus callosum, brainstem, and cerebellum). We used the Rutherford classification20 to evaluate the injury severity in the four regions of interest as initially described: normal, mild, moderate, or severe BGT, WM and cortex injuries; equivocal or abnormal PLIC signal. We adapted this classification for three supplementary regions: corpus callosum (splenium/knee), brainstem (midbrain/pons/medulla), and cerebellum (hemisphere/vermis). Mild-moderate injury was defined as the involvement of one sub-location for the small structures: corpus callosum and brainstem. For example, a single injury of splenium or pons was considered as mild-moderate; an injury including splenium and knee for the corpus callosum or midbrain, and pons for brainstem was considered as severe. For the cerebellum, a unilateral injury of a hemisphere or of the vermian lonely was considered as mild-moderate. Vermian injury associated with unilateral or bilateral hemispheric injury, was considered severe.
The single or multiple locations of brain injuries were defined according to the number of injured regions of interest. Multiple brain injury was defined as injury present in more than one region of interest (i.e., BGT, cortex, WM).
For infants who underwent MRI several times before 12 days of life, only the MRI scan with the most severe findings was taken into account.
MRIs performed after 12 days of life were not used in this study because the average time to perform these MRIs (25 ± 22 days), the indication and expected information did not seem comparable to those performed before 12 days of life.
Of note, no common national HIE-TH imaging protocol was available during the study. MRI was performed at the discretion of the physician.
Outcomes at discharge
Neurological examination at discharge was evaluated according to 7 items: consciousness, axial and peripheral tone, spontaneous motor skills, posture, sucking and pupillary reflex. For each item, 3 qualifications were proposed: normal, moderate, and severe. All items had to be normal for the examination to be considered normal.
Data management and statistics
The characteristics of the two groups of infants with and without MRI were compared. MRI scan timing and sequences were described. The severity of the injuries in the seven regions of interest and the single or combined characteristics of injuries in the three main regions (BGT, WM, and cortex) were described. Finally, the association between brain patterns and the short-term outcome was reported.
Data are described with a mean (±standard deviation) for quantitative variables and number (percentage) for categorical variables. Groups were compared by Fisher exact test for categorical variables and Mann–Whitney U test for quantitative variables. All tests were 2-sided; P < 0.05 was considered statistically significant. Statistical analyses involved using Intercooled STATA v16 (Stata Corp., College Station, TX).
Ethics
Informed consent was obtained from both parents. The study protocol was approved by the Advisory National Committee on the treatment of personal health data for research purposes (Comité Consultatif sur le Traitement de l’Information en matière de Recherche sur la Santé, reference no. 14.724). Authorizations were obtained from the National French data protection authority (Commission Nationale Informatique et Libertés, DR-2015-136) and the regional ethics committee (Comité de Protection des Personnes Sud Est; Institutional Review Board no. 5891).
Results
Population
Among 794 newborns enrolled in LyTONEPAL, 575 were eligible for the study and 479 underwent MRI, performed in 57 of the 61 participating centers (Fig. 1). Infants without MRI data had the poorest neonatal adaptation, showed more severe NE, and had a higher rate of death as compared with infants with MRI data (Table 1). The time to reach 34 °C was similar in the two groups and was 6.0 ± 4.3 h for the group with MRI.
wg weeks’ gestation, NE neonatal encephalopathy, grade I–II–III according to ref. 19 TH therapeutic hypothermia, D day.
French brain MRI practices
All living infants included in this study had at least one MRI between 0 and 12 days of life. Of note, a majority of them (337 (N = 479, 70%)), underwent MRI between 4 and 7 days of life. Both DWI and conventional sequences were performed in more than 95% of MRI scans (Fig. 2). The MRI quality was considered good or excellent for 441 scans (92.1%), poor for 21 (4.4%), and unknown for 17 (3.5%).
In total, 450 (N = 479, 93.9%) infants underwent a single MRI and 29 had two MRI scans within the first 12 days of life. Among these 29 infants, 26 had concordant MRI results (12 with normal MRI and 14 with brain injuries). Three infants had a slightly different interpretation (for one infant, the most severe MRI image was the first MRI on day 4 (severe WM injury), and for the two other infants, the second on days 7 and 8 was the most severe (mild-moderate BGT and WM injuries for one, mild-moderate WM and cortex injuries for the second)).
Injury patterns and their incidence
MRI findings were normal for 231 (N = 479, 48.2%) infants. Among the remaining 248 (N = 479, 51.8%) infants, 242 had brain injuries located in BGT (n = 157, 63.3%), WM (n = 148, 59.7%) and cortex (n = 114, 46.0%). Four other injury locations were observed: PLIC (n = 72, 29.0%), corpus callosum (n = 56, 22.6%), brainstem (n = 39, 15.7%), and cerebellum (n = 23, 9.3%). The injury severity is detailed for each region of interest, BGT, WM, cortex and PLIC, corpus callosum, brainstem and cerebellum (see “Methods” section) in Fig. 3 and Supplementary data 2. The distribution between mild-moderate and severe injuries was approximately balanced for all regions except for severely damaged PLIC for two-thirds of the infants.
BGT basal ganglia thalami, WM white matter, PLIC posterior-limb internal capsule. Injuries of BGT, WM, cortex, and PLIC were defined according to Rutherford classification:8, 20 normal (0), mild (1), and moderate (2) combined in a single grade, severe (3), except for PLIC with mild-moderate=unilateral and severe=bilateral. Corpus callosum: splenium/knee, brainstem: midbrain/pons/medulla, cerebellum: hemisphere/vermis, for each, was defined as normal (0), mild-moderate (1) as the involvement of one item, and severe (2) as the involvement of more than one item.
For 155 infants (N = 248, 62.5%), brain injuries were reported in more than one region (multiple injuries). The association of the three main injured structures are detailed in Fig. 4.
PLIC, corpus callosum, brainstem and cerebellum injuries were mainly associated with the three predominant structures: PLIC (n = 66; 91.7%) and brainstem (n = 37, 94.9%) with BGT; corpus callosum (n = 49, 87.5%) with cortex; cerebellum (n = 18, 78.3%) with WM. Atleast 57 single BGT injuries (23.0%) and 14 single cortex injuries (5.6%) were observed.
MRI patterns and short-term outcome
Associations of MRI patterns with the short-term outcome are detailed in Table 2. The clinical examination at discharge was performed at a mean of 16 ± 11 days. Infants with normal MRI or a single injury had the lowest death rate and the highest normal clinical examination scores at discharge. Of note, infants with MRI-evidenced WM and cortex injuries had a similar outcome compared to infants with BGT single injury. Infants with combined cerebral injuries, especially when BGT, cortex, and WM lesions were associated, had a higher death rate (63.3%, 95% CI 48.3–76.6). Surviving infants with this MRI pattern frequently had an abnormal examination at discharge (20.4%, 95% CI 10.2–34.3).
Discussion
This prospective population-based cohort detailed the practice of neuroimaging in France and the panorama of brain injury patterns observed in infants in a context of NE treated by TH. MRI scans (70%) were performed soon after TH between 4 and 7 days after birth. Notably, 48.2% of the infants had normal MRI findings. Among the brain-injured infants, the most frequent injured structures were BGT, WM, and cortex. Single structure injuries were rare. Most of the infants with normal MRI or a single injured structure (WM or cortex) survived and had a normal clinical examination at discharge.
MRI practices in NE
In 2014, the American College of Obstetricians and Gynecologists recommended performing early and late MRI between 24 and 96 h and 7 and 21 days of life, respectively, to delineate the timing of the perinatal cerebral injury and the full extent of cerebral injury.21 Recent studies report that early MRI with DWI sequences could be reliable to investigate brain injury and improve patient care.22,23,24
Our study showed different practices: a single MRI was mostly performed between 4 and 7 days of life, just after the rewarming. These MRI sessions were performed with adequate sequences including DWI and conventional T1–T2 sequences. The quality of the images was evaluated as very good by radiologists in most cases.
Despite these previous recommendations, there is still ongoing debate as to the optimal timing and conditions of MRI for NE patients.3,8,22,25 With DWI sequences and TH implementation, MRI is performed earlier, during the first days of life. Several authors have observed good agreement between DWI sequences in early MRI and conventional T1–T2 sequences in late MRI.22,23 Our results do not disprove these findings, showing non-significant differences for the infants who underwent MRI twice. The large implementation of DWI sequences and the improved experience of early MRI reading with DWI sequences could explain the change in practice observed in this current cohort. No common imaging protocol for NE-HIE exists in France, nor in others countries despite the multiple recommendations found in the literature.26,27,28 The lack of common protocol maybe because that access to MRI varies widely across centers and according to whether individual patient conditions require rapid information or not. Clearly, the 4–-7 days timing appears as the ideal window of opportunity for NE-HIE patients in France.
Characteristics of MRI patterns in the era of TH
A notable finding of this present study was the high rate of normal MRI findings. Some authors reported variable rates for normal MRI under hypothermic conditions ranging from 17 to 62% (12–89 patients).15,29,30,31 In a similar way, BGT injury frequency varied notably in the literature (10–60%), and we observed a higher frequency (63.3%) in our study when infants had cerebral injuries.15,32 These discrepancies might be explained by differences between the studies in term of the maternity level of care, patient recruitment, normo- or hypothermic conditions, the number of inclusions and participative centers, gestational age (preterm infant enrollment), MRI timing, and year of publication (technological improvement). Finally, the distribution of WM and cortex injuries in our study agrees with the literature.15,31,33,34
Combined injuries were observed for most of the newborns with abnormal MRI findings. These injury patterns were similarly reported in the literature.5,9,10,11 This result may suggest that NE due to a hypoxic–ischemic event is a diffuse disease.
In our cohort, injuries of the corpus callosum and cerebellum were observed but were not predominant. These injuries were reported almost exclusively in association with cortical or WM injuries. In the literature, few reports described infra-tentorial and corpus callosum injuries, except for secondary cerebellum atrophy seen several weeks or months after the initial brain injury.35,36,37 Corpus callosum injury should be considered a secondary axonal disruption due to WM damage. The nature of cerebellum injuries was not detailed by radiologists in our study.
The cerebellum and corpus callosum should be identified as possible injury locations in NE resulting from HIE in the early neonatal period.
MRI pattern and outcome at discharge
All but one infant with normal MRI findings survived, and 68.4% of them had a normal examination at discharge. The outcome of patients with a single WM or cortex injury was quite similar. Conversely, patients with more than one brain injury structure had a higher rate of death or abnormal examination at discharge. Indeed, when the three predominant injured structures (BGT, WM, cortex) were combined, the death rate reached 50%. This data needs to be interpreted with caution as a death in this context often occurred after a redirection of care.
Clinical examination at discharge did not reflect MRI findings. Brain functions are not mature at birth and the sensitivity of clinical examination will increase with age.38,39 Timing to the last sedative drug administration from discharge, parental depression influencing interaction, post-natal age of the infant at discharge may also have interfered. A validated neonatal clinical examination grid and a parental assessment such as the Brazelton scale40 were not carried out, which may explain this result. Furthermore, MRI provided anatomical but not functional information. Correlation with the neurological outcome at 18 months and 3 years is needed to conclude definitively about the prognostic value of both the clinical examination at discharge and the MRI finding.
In the literature, cerebral injury is associated with an unfavorable prognosis.41 Kasdorf et al. observed death or neurodevelopmental abnormalities (spastic quadriplegia, cognitive delay) at age 3 years for 88% of infants with BGT injury.13 Shankaran et al. observed a strong association between MRI severity score and outcome,42 with good predictive value as well as good sensitivity and specificity of MRI for neurodevelopmental outcome at age 6–7 years.28
Long-term follow-up of the LyTONEPAL cohort will provide valuable information concerning the relation between structural injuries, discharge examination and long-term neurodevelopmental outcome.
Strengths and limitations
This study was a population-based cohort representative of the French experience in NE several years after the implementation of TH in France. This is the largest multicentric cohort study designed for MRI to investigate neonates treated with hypothermia. The standardization of the MRI protocol, a structured report, and radiologist experience in French level III maternity units ensured the quality of results. The classification in seven brain regions, including the description of the infra-tentorial injuries, provided updated data.
Nevertheless, the MRI data obtained from multiple centers, without centralized reading, may have introduced bias. The patients without MRI differed from the study group in terms of disease severity, and thus their poor neonatal adaptation and a higher rate of death could imply selection bias. The discharge neurological exam in LyTONEPAL study was standardized (tone, alertness, sucking, and pupillary reflex), but as we know, the clinical exam is ultimately dependent on the experience and the insight of the physician, which may influence the reproducibility of the results.
Conclusion
This study updates the panorama of brain MRI injury patterns in hypoxic–ischemic newborns evaluated within one week after birth in the era of therapeutic hypothermia, confirming the large predominance of basal ganglia/thalami injuries and the existence of other less identified locations such as the cerebellum and corpus callosum Half of the infants had normal findings and less than a quarter of the population had multiple injuries on MRI, thus suggesting that therapeutic hypothermia may reduce brain injuries. Correlation with the neurodevelopmental outcomes is needed.
With improved sequences and techniques in detecting brain injury, MRI is now performed earlier, between 4 and 7 days, depending on of the clinical situation of the newborn.
The clinical examination at discharge did not reflect MRI findings because the clinicians did not use the assessment grid which included standardized items.
Follow-up of these patients is in progress and will provide data to validate the MRI prognostic value.
References
Pierrat, V. et al. Prevalence, causes, and outcome at 2 years of age of newborn encephalopathy: population based study. Arch. Dis. Child. Fetal Neonatal Ed. 90, F257–F261 (2005).
Kurinczuk, J. J., White-Koning, M. & Badawi, N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum. Dev. 86, 329–338 (2010).
Weeke, L. C. et al. A novel magnetic resonance imaging score predicts neurodevelopmental outcome after perinatal asphyxia and therapeutic hypothermia. J. Pediatr. 192, 33–40.e2 (2018).
Bednarek, N. et al. Impact of therapeutic hypothermia on MRI diffusion changes in neonatal encephalopathy. Neurology 78, 1420–1427 (2012).
Okereafor, A. et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 121, 906–914 (2008).
van Kooij, B. J. M. et al. Serial MRI and neurodevelopmental outcome in 9- to 10-year-old children with neonatal encephalopathy. J. Pediatr. 157, 221–227.e2 (2010).
Martinez-Biarge, M. et al. White matter and cortical injury in hypoxic-ischemic encephalopathy: antecedent factors and 2-year outcome. J. Pediatr. 161, 799–807 (2012).
Rutherford, M. et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic–ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol. 9, 39–45 (2010).
Inder, T. E. et al. Randomized trial of systemic hypothermia selectively protects the cortex on MRI in term hypoxic-ischemic encephalopathy. J. Pediatr. 145, 835–837 (2004).
Miller, S. P. et al. Patterns of brain injury in term neonatal encephalopathy. J. Pediatr. 146, 453–460 (2005).
Rao, S. et al. Long-term outcomes and risk factors associated with acute encephalitis in children. J. Pediatr. Infect. Dis. Soc. https://doi.org/10.1093/jpids/piv075 (2015).
Barnett, A. et al. Neurological and perceptual-motor outcome at 5 - 6 years of age in children with neonatal encephalopathy: relationship with neonatal brain MRI. Neuropediatrics 33, 242–248 (2002).
Kasdorf, E., Engel, M., Heier, L. & Perlman, J. M. Therapeutic hypothermia in neonates and selective hippocampal injury on diffusion-weighted magnetic resonance imaging. Pediatr. Neurol. 51, 104–108 (2014).
Kuenzle, C. et al. Prognostic value of early MR imaging in term infants with severe perinatal asphyxia. Neuropediatrics 25, 191–200 (1994).
Walsh, B. H. et al. The frequency and severity of magnetic resonance imaging abnormalities in infants with mild neonatal encephalopathy. J. Pediatr. 187, 26–33.e1 (2017).
Jacobs, S. E. et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. CD003311. https://doi.org/10.1002/14651858.CD003311.pub3 (2013).
Saliba, E. & Debillon, T. Hypothermia for hypoxic-ischemic encephalopathy in fullterm newborns. Arch. Pediatr. Organe . Soc. Francaise Pediatr. 17(Suppl 3), S67–S77 (2010).
Debillon, T., Bednarek, N. & Ego, A. LyTONEPAL: long term outcome of neonatal hypoxic encephalopathy in the era of neuroprotective treatment with hypothermia: a French population-based cohort. BMC Pediatr. 18, 255 (2018).
Sarnat, H. B. & Sarnat, M. S. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch. Neurol. 33, 696–705 (1976).
Mercuri, E. et al. Head growth in infants with hypoxic-ischemic encephalopathy: correlation with neonatal magnetic resonance imaging. Pediatrics 106, 235–243 (2000).
D’Alton, M. E. et al. Executive summary: Neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of Obstetricians and Gynecologists’ Task Force on Neonatal Encephalopathy. Obstet. Gynecol. 123, 896–901 (2014).
Agut, T. et al. Early identification of brain injury in infants with hypoxic ischemic encephalopathy at high risk for severe impairments: accuracy of MRI performed in the first days of life. BMC Pediatr. 14, 177 (2014).
Charon, V. et al. Comparison of early and late MRI in neonatal hypoxic-ischemic encephalopathy using three assessment methods. Pediatr. Radiol. 45, 1988–2000 (2015).
Charon, V. et al. Early MRI in neonatal hypoxic-ischaemic encephalopathy treated with hypothermia: prognostic role at 2-year follow-up. Eur. J. Radiol. 85, 1366–1374 (2016).
Barkovich, A. J. et al. Prediction of neuromotor outcome in perinatal asphyxia: evaluation of MR scoring systems. AJNR 19, 143–149 (1998).
Azzopardi, D. et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr. 8, 17 (2008).
Shankaran, S. et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N. Engl. J. Med 353, 1574–1584 (2005).
Shankaran, S. et al. Neonatal magnetic resonance imaging pattern of brain injury as a biomarker of childhood outcomes following a trial of hypothermia for neonatal hypoxic-ischemic encephalopathy. J. Pediatr. 167, 987–993.e3 (2015).
Guillot, M. et al. Influence of timing of initiation of therapeutic hypothermia on brain MRI and neurodevelopment at 18 months in infants with HIE: a retrospective cohort study. BMJ Paediatr. Open 3, e000442 (2019).
Shankaran, S. et al. Childhood outcomes after hypothermia for neonatal encephalopathy. N. Engl. J. Med. 366, 2085–2092 (2012).
Shapiro, K. A. et al. Early changes in brain structure correlate with language outcomes in children with neonatal encephalopathy. NeuroImage Clin. 15, 572–580 (2017).
Tusor, N. et al. Prediction of neurodevelopmental outcome after hypoxic-ischemic encephalopathy treated with hypothermia by diffusion tensor imaging analyzed using tract-based spatial statistics. Pediatr. Res. 72, 63–69 (2012).
Cheong, J. L. Y. et al. Prognostic utility of magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: substudy of a randomized trial. Arch. Pediatr. Adolesc. Med. 166, 634–640 (2012).
De Wispelaere, L. A. et al. Electroencephalography and brain magnetic resonance imaging in asphyxia comparing cooled and non-cooled infants. Eur. J. Paediatr. Neurol. 23, 181–190 (2019).
Sargent, M. A., Poskitt, K. J., Roland, E. H., Hill, A. & Hendson, G. Cerebellar vermian atrophy after neonatal hypoxic-ischemic encephalopathy. AJNR 25, 1008–1015 (2004).
Le Strange, E., Saeed, N., Cowan, F. M., Edwards, A. D. & Rutherford, M. A. MR imaging quantification of cerebellar growth following hypoxic-ischemic injury to the neonatal brain. AJNR 25, 463–468 (2004).
Annink, K. V. et al. Cerebellar injury in term neonates with hypoxic-ischemic encephalopathy is underestimated. Pediatr. Res. https://doi.org/10.1038/s41390-020-01173-z (2020).
Murray, D. M. et al. The predictive value of early neurological examination in neonatal hypoxic-ischaemic encephalopathy and neurodevelopmental outcome at 24 months. Dev. Med. Child Neurol. 52, e55–e59 (2010).
Grass, B. et al. Short-term neurological improvement in neonates with hypoxic-ischemic encephalopathy predicts neurodevelopmental outcome at 18–24 months. J. Perinat. Med. 48, 296–303 (2020).
Brazelton, T. B. The Brazelton Neonatal Behavior Assessment Scale: introduction. Monogr. Soc. Res. Child Dev. 43, 1–13 (1978).
Sánchez Fernández, I., Morales-Quezada, J. L., Law, S. & Kim, P. Prognostic value of brain magnetic resonance imaging in neonatal hypoxic-ischemic encephalopathy: a meta-analysis. J. Child Neurol. 32, 1065–1073 (2017).
Shankaran, S. et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch. Dis. Child. Fetal Neonatal Ed. 97, F398–F404 (2012).
Acknowledgements
We thank members of the LyTONEPAL Study Group and all the regional teams participating in the study and all French maternity and neonatal units for their substantial contribution to the acquisition of data; Laura Smales and Grace Stockton for their helpful contribution to proofreading. We are grateful for the participation of all families of infants in the LyTONEPAL cohort study. We thank the American Memorial Committee for their considerable support. This study was funded by the National Program for Clinical Research (PHRC-N-13-0327).
The LyTONEPAL Study Group Collaborators
Auvergne Rhone Alpes: N. Bouchon-Guedj (Chambéry), G. Remerand (Clermont-Ferrand), M. Chevallier (Grenoble), O. Claris (Lyon, HFME), C.M. Loys (Lyon, Croix Rousse), H. Patural (Saint-Etienne), Bourgogne Franche Comté: T. Dabudyk (Besançon), C. Chantegret (Dijon), Bretagne: J.M. Roué (Brest), M. Gromand (Rennes), A. Busnel (St-Brieuc), A. Sevestre (Vannes), Centre Val de Loire: J. Guerreiro (Orléans), G. Favrais (Tours), Grand Est: J. Nakhleh (Mulhouse), N. Bednarek (Reims), D. Astruc, (Strasbourg), B. Kassis-Makhoul (Troyes), Hauts de France: G. Ghostine (Amiens), J. Ghesquiere (Arras), L. Egreteau (Calais), S.M. Dhahbi (Creil), S. Klosowski (Lens), F. Flamein (Lille), J. Balitalike (Valenciennes), Ile-de-France: D. Brau (Argenteuil), V. Zupan-Simunek (Clamart), C. Huon (Colombes), M. Tauzin (Créteil), M. Merhi (Evry), N. Le Sache (Le Kremlin-Bicêtre), B. Heller Roussin (Montreuil), D. Mellah (Meaux), A. Lapillonne, E. Leroy Terquem (Paris, Necker), J. Patkai (Paris, Port Royal), V. Biran (Paris, Robert Debré) I. Guellec (Paris, Trousseau), A. Durandy (Poissy), P. Boize (Pontoise), F. Goudjil (St Denis), Nouvelle Aquitaine: P. Jouvencel (Bayonne), O. Brissaud (Bordeaux), F. Mons (Limoges), K. Norbert (Pau), A. Parizel (Poitiers), Occitanie: G. Cambonie (Montpellier), M. Di Maio (Nîmes), R. Salloum (Perpignan), M.O. Marcoux (Toulouse), Pays de Loire: S. Le Bouedec (Angers), C. Flamant (Nantes), Y. Montcho (Le Mans), Provence Alpes Côte d’Azur: C. Desrobert (Marseille La Conception), V. Brevaut-Malaty (Marseille, Nord), F. Casagrande (Nice), R. Salloum (Perpignan), Martinique: S. Ketterer Martinon (Fort de France), Normandie: A. Cénéric (Caen), J. Mourdie (Le Havre), A. Chadie (Rouen), La Réunion: M. Carbonnier (Saint-Pierre), D. Ramful (Saint-Denis).
Author information
Authors and Affiliations
Contributions
J.B., T.D., A.V., C.L., A.E., and P.-Y.A. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. J.B., T.D., N.B., V.P., A.E., and P.-Y.A. conceptualized the study and wrote the manuscript. J.B. and A.V. performed the statistical analysis. C.L., A.V., M.A., and L.-H.P. coordinated data collection and had responsibility for technical support. T.D. obtained funding and supervised the study. All authors contributed to the data analysis and interpretation of the results and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Consent statement
Informed consent was obtained from both parents. The study protocol was approved by the Advisory National Committee on the treatment of personal health data for research purposes (Comité Consultatif sur le Traitement de l’Information en matière de Recherche sur la Santé, reference no. 14.724). Authorizations were obtained from the National French data protection authority (Commission Nationale Informatique et Libertés, DR-2015-136) and the regional ethics committee (Comité de Protection des Personnes Sud Est; Institutional Review Board no. 5891).
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Beck, J., Bednarek, N., Pierrat, V. et al. Cerebral injuries in neonatal encephalopathy treated with hypothermia: French LyTONEPAL cohort. Pediatr Res 92, 880–887 (2022). https://doi.org/10.1038/s41390-021-01846-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-021-01846-3