Abstract
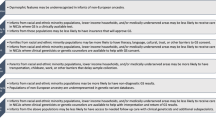
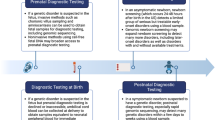
Rapid genomic sequencing has been shown to have a high diagnostic yield for critically ill infants, with multiple research studies demonstrating both diagnostic and clinical utility. However, clinical implementation of rapid sequencing in the neonatal intensive care unit (NICU), as well as other aspects of genomic medicine such as precision therapy, may be challenging. We describe the Neonatal Genomics Program, developed at our institution as a multidisciplinary approach to improve clinical genetic diagnosis and outcomes for infants in our NICU through genomic medicine. The creation of a dedicated program implementing genomic medicine to improve care in the NICU allows not only for improved access to genomic sequencing for rapid diagnosis, but also advancement of rare disease research and precision therapeutics. Ongoing efforts will help to define an optimal approach to genomic medicine in the NICU context.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
No new data were generated for this study.
References
Gubbels CS, VanNoy GE, Madden JA, Copenheaver D, Yang S, Wojcik MH, et al. Prospective, phenotype-driven selection of critically ill neonates for rapid exome sequencing is associated with high diagnostic yield. Genet Med. 2020;22:736–44.
Elliott AM, du Souich C, Lehman A, Guella I, Evans DM, Candido T, et al. RAPIDOMICS: rapid genome-wide sequencing in a neonatal intensive care unit-successes and challenges. Eur J Pediatr. 2019;178:1207–18.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017:e173438.
van Diemen CC, Kerstjens-Frederikse WS, Bergman KA, de Koning TJ, Sikkema-Raddatz B, van der Velde JK, et al. Rapid targeted genomics in critically ill newborns. Pediatrics 2017;140:2854.
Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in Ill infants. Am J Hum Genet. 2019;105:719–33.
Wojcik MH, Del Rosario MC, Agrawal PB. Perspectives of United States neonatologists on genetic testing practices. Genet Med. 2022;24:1372–7.
Robinson JO. Ask me later: deciding to have clinical exome trio sequencing for my critically ill child. Genet Med. 2021;23:1836–7.
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–63.
Callahan KP, Flibotte J, Skraban C, Wild KT, Joffe S, Munson D, et al. How neonatologists use genetic testing: findings from a national survey. J Perinatol. 2022;42:260–1.
Best S, Brown H, Lunke S, Patel C, Pinner J, Barnett CP, et al. Learning from scaling up ultra-rapid genomic testing for critically ill children to a national level. NPJ Genom Med. 2021;6:5.
Dimmock DP, Clark MM, Gaughran M, Cakici JA, Caylor SA, Clarke C, et al. An RCT of rapid genomic sequencing among seriously Ill infants results in high clinical utility, changes in management, and low perceived harm. Am J Hum Genet. 2020;107:942–52.
Maron JL, Kingsmore SF, Wigby K, Chowdhury S, Dimmock D, Poindexter B, et al. Novel variant findings and challenges associated with the clinical integration of genomic testing: an interim report of the genomic medicine for Ill Neonates and Infants (GEMINI) Study. JAMA Pediatr. 2021;175:e205906.
Manickam K, McClain MR, Demmer LA, Biswas S, Kearney HM, Malinowski J, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med 2021;23:2029–37.
Morton SU, Christodoulou J, Costain G, Muntoni F, Wakeling E, Wojcik MH, et al. Multicenter consensus approach to evaluation of neonatal hypotonia in the genomic era: a review. JAMA Neurol. 2022;79:405–13.
Franck LS, Kriz RM, Rego S, Garman K, Hobbs C, Dimmock D. Implementing rapid whole-genome sequencing in critical care: a qualitative study of facilitators and barriers to new technology adoption. J Pediatr. 2021;237:237–43.
Ceyhan-Birsoy O, Murry JB, Machini K, Lebo MS, Yu TW, Fayer S, et al. Interpretation of genomic sequencing results in healthy and ill newborns: results from the BabySeq Project. Am J Hum Genet. 2019;104:76–93.
Schmitz-Abe K, Li Q, Rosen SM, Nori N, Madden JA, Genetti CA, et al. Unique bioinformatic approach and comprehensive reanalysis improve diagnostic yield of clinical exomes. Eur J Hum Genet. 2019;27:1398–405.
Wojcik MH, Schwartz TS, Thiele KE, Paterson H, Stadelmaier R, Mullen TE, et al. Infant mortality: the contribution of genetic disorders. J Perinatol. 2019;39:1611–9.
Rockowitz S, LeCompte N, Carmack M, Quitadamo A, Wang L, Park M, et al. Children's rare disease cohorts: an integrative research and clinical genomics initiative. NPJ Genom Med. 2020;5:29.
Wojcik MH, Stewart JE, Waisbren SE, Litt JS. Developmental support for infants with genetic disorders. Pediatrics 2020;145:e20190629.
Grosse SD, Farnaes L. Genomic sequencing in acutely ill infants: what will it take to demonstrate clinical value? Genet Med. 2019;21:269–71.
Zahed H, Sparks TN, Li B, Alsadah A, Shieh JTC. Potential role of genomic sequencing in the early diagnosis of treatable genetic conditions. J Pediatr. 2017;189:222–6.
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl J Med. 2017;377:1723–32.
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N. Engl J Med. 2019;381:1644–52.
Funding
MHW is supported by K23 HD102589. AMD is supported by T32 HD098061. PBA is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, R01AR068429-01, National Human Genome Research Institute, 1R01HG011798-01A1 and the “Because of Bella” foundation.
Author information
Authors and Affiliations
Contributions
MHW, AMD, and PBA contributed to conceptualization, methodology, writing of the original draft, and review and editing.
Corresponding authors
Ethics declarations
Competing interests
PBA is a member of the Scientific Advisory Board of Illumina, Inc and GeneDx, Inc. All other authors declare no conflicts of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wojcik, M.H., D’Gama, A.M. & Agrawal, P.B. A model to implement genomic medicine in the neonatal intensive care unit. J Perinatol 43, 248–252 (2023). https://doi.org/10.1038/s41372-022-01428-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-022-01428-z
This article is cited by
-
Role of genomic medicine and implementing equitable access for critically ill infants in neonatal intensive care units
Journal of Perinatology (2023)
-
Genomic medicine in neonatal care: progress and challenges
European Journal of Human Genetics (2023)