Abstract
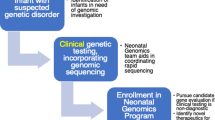
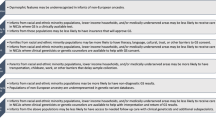
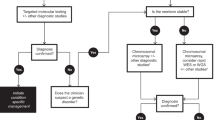
During the neonatal period, many genetic disorders present and contribute to neonatal morbidity and mortality. Genomic medicine—the use of genomic information in clinical care— has the potential to significantly reduce morbidity and mortality in the neonatal period and improve outcomes for this population. Diagnostic genomic testing for symptomatic newborns, especially rapid testing, has been shown to be feasible and have diagnostic and clinical utility, particularly in the short-term. Ongoing studies are assessing the feasibility and utility, including personal utility, of implementation in diverse populations. Genomic screening for asymptomatic newborns has also been studied, and the acceptability and feasibility of such an approach remains an active area of investigation. Emerging precision therapies, with examples even at the “n-of-1” level, highlight the promise of precision diagnostics to lead to early intervention and improve outcomes. To sustainably implement genomic medicine in neonatal care in an ethical, effective, and equitable manner, we need to ensure access to genetics and genomics knowledge, access to genomic tests, which is currently limited by payors, feasible processes for ordering these tests, and access to follow up in the clinical and research realms. Future studies will provide further insight into enablers and barriers to optimize implementation strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, et al. Exome sequencing identifies the cause of a mendelian disorder. Nat Genet. 2010;42:30–5.
Genomics Education Programme. Genomic medicine. 2021. https://www.genomicseducation.hee.nhs.uk/glossary/genomic-medicine/.
National Human Genome Research Institute. Genomics and medicine. 2020. https://www.genome.gov/health/Genomics-and-Medicine.
Kingsmore SF, Cole FS. The role of genome sequencing in neonatal intensive care units. Annu Rev Genomics Hum Genet. 2022;23:427–48.
Saunders CJ, Miller NA, Soden SE, Dinwiddie DL, Noll A, Alnadi NA, et al. Rapid whole-genome sequencing for genetic disease diagnosis in neonatal intensive care units. Sci Transl Med. 2012;4:154ra35.
Almli LM, Ely DM, Ailes EC, Abouk R, Grosse SD, Isenburg JL, et al. Infant mortality attributable to birth defects - United States, 2003-2017. MMWR Morb Mortal Wkly Rep. 2020;69:25–9.
Heron M. Deaths: leading causes for 2019. Natl Vital Stat Rep. 2021;70:1–114.
Cuna A, George L, Sampath V. Genetic predisposition to necrotizing enterocolitis in premature infants: current knowledge, challenges, and future directions. Semin Fetal Neonatal Med. 2018;23:387–93.
D’Gama AM, Del Rosario MC, Bresnahan MA, Yu TW, Wojcik MH, Agrawal PB. Integrating rapid exome sequencing into NICU clinical care after a pilot research study. NPJ Genom Med. 2022;7:51.
Australian Genomics Health Alliance Acute Care F, Lunke S, Eggers S, Wilson M, Patel C, Barnett CP, et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian Public Health Care System. JAMA. 2020;323:2503–11.
Bowling KM, Thompson ML, Finnila CR, Hiatt SM, Latner DR, Amaral MD, et al. Genome sequencing as a first-line diagnostic test for hospitalized infants. Genet Med. 2022;24:851–61.
Dimmock D, Caylor S, Waldman B, Benson W, Ashburner C, Carmichael JL, et al. Project Baby Bear: Rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am J Hum Genet. 2021;108:1231–8.
Dimmock DP, Clark MM, Gaughran M, Cakici JA, Caylor SA, Clarke C, et al. An RCT of rapid genomic sequencing among seriously Ill infants results in high clinical utility, changes in management, and low perceived harm. Am J Hum Genet. 2020;107:942–52.
French CE, Delon I, Dolling H, Sanchis-Juan A, Shamardina O, Megy K, et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45:627–36.
NICUSeq Study Group, Krantz ID, Medne L, Weatherly JM, Wild KT, Biswas S, et al. Effect of whole-genome sequencing on the clinical management of acutely Ill infants with suspected genetic disease: a randomized clinical trial. JAMA Pediatr. 2021;175:1218–26.
Gubbels CS, VanNoy GE, Madden JA, Copenheaver D, Yang S, Wojcik MH, et al. Prospective, phenotype-driven selection of critically ill neonates for rapid exome sequencing is associated with high diagnostic yield. Genet Med. 2020;22:736–44.
Kingsmore SF, Cakici JA, Clark MM, Gaughran M, Feddock M, Batalov S, et al. A randomized, controlled trial of the analytic and diagnostic performance of singleton and trio, rapid genome and exome sequencing in Ill infants. Am J Hum Genet. 2019;105:719–33.
Maron JL, Kingsmore SF, Wigby K, Chowdhury S, Dimmock D, Poindexter B, et al. Novel variant findings and challenges associated with the clinical integration of genomic testing: an interim report of the genomic medicine for Ill neonates and infants (GEMINI) study. JAMA Pediatr. 2021;175:e205906.
Meng L, Pammi M, Saronwala A, Magoulas P, Ghazi AR, Vetrini F, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438.
Petrikin JE, Cakici JA, Clark MM, Willig LK, Sweeney NM, Farrow EG, et al. The NSIGHT1-randomized controlled trial: rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018;3:6.
Stark Z, Tan TY, Chong B, Brett GR, Yap P, Walsh M, et al. A prospective evaluation of whole-exome sequencing as a first-tier molecular test in infants with suspected monogenic disorders. Genet Med. 2016;18:1090–6.
Willig LK, Petrikin JE, Smith LD, Saunders CJ, Thiffault I, Miller NA, et al. Whole-genome sequencing for identification of Mendelian disorders in critically ill infants: a retrospective analysis of diagnostic and clinical findings. Lancet Respir Med. 2015;3:377–87.
Manickam K, McClain MR, Demmer LA, Biswas S, Kearney HM, Malinowski J, et al. Exome and genome sequencing for pediatric patients with congenital anomalies or intellectual disability: an evidence-based clinical guideline of the American College of Medical Genetics and Genomics (ACMG). Genet Med. 2021;23:2029–37.
Wojcik MH, D’Gama AM, Agrawal PB. A model to implement genomic medicine in the neonatal intensive care unit. J Perinatol. 2023;43:248–52.
Owen MJ, Niemi AK, Dimmock DP, Speziale M, Nespeca M, Chau KK, et al. Rapid sequencing-based diagnosis of thiamine metabolism dysfunction syndrome. N Engl J Med. 2021;384:2159–61.
Diamonstein C, Stevens B, Shahrukh Hashmi S, Refuerzo J, Sullivan C, Hoskovec J. Physicians’ awareness and utilization of genetic services in Texas. J Genet Couns. 2018;27:968–77.
Haga SB, Kim E, Myers RA, Ginsburg GS. Primary care physicians’ knowledge, attitudes, and experience with personal genetic testing. J Pers Med. 2019;9:29.
Hawkins AK, Hayden MR. A grand challenge: providing benefits of clinical genetics to those in need. Genet Med. 2011;13:197–200.
Jenkins BD, Fischer CG, Polito CA, Maiese DR, Keehn AS, Lyon M, et al. The 2019 US medical genetics workforce: a focus on clinical genetics. Genet Med. 2021;23:1458–64.
Lee G, Yu L, Suarez CJ, Stevenson DA, Ling A, Killer L. Factors associated with the time to complete clinical exome sequencing in a pediatric patient population. Genet Med. 2022;24:2028–33.
Suther S, Kiros GE. Barriers to the use of genetic testing: a study of racial and ethnic disparities. Genet Med. 2009;11:655–62.
DeBerardinis RJ, Keshari KR. Metabolic analysis as a driver for discovery, diagnosis, and therapy. Cell. 2022;185:2678–89.
Wojcik MH, Reimers R, Poorvu T, Agrawal PB. Genetic diagnosis in the fetus. J Perinatol. 2020;40:997–1006.
Adams S, Llorin H, Dobson LJ, Studwell C, Wilkins-Haug L, Guseh S, et al. Postnatal genetic testing on cord blood for prenatally identified high-probability cases. Prenat Diagn. 2023;43:1120–31.
Almannai M, Marom R, Sutton VR. Newborn screening: a review of history, recent advancements, and future perspectives in the era of next generation sequencing. Curr Opin Pediatr. 2016;28:694–9.
Wilson J, Jungner G, WHO. Principles and practice of screening for disease. World Health Organization, 1968.
Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel. 2023. https://www.hrsa.gov/advisory-committees/heritable-disorders/rusp.
Baker MW, Groose M, Hoffman G, Rock M, Levy H, Farrell PM. Optimal DNA tier for the IRT/DNA algorithm determined by CFTR mutation results over 14 years of newborn screening. J Cyst Fibros. 2011;10:278–81.
Ding Y, Owen M, Le J, Batalov S, Chau K, Kwon YH, et al. Scalable, high quality, whole genome sequencing from archived, newborn, dried blood spots. NPJ Genom Med. 2023;8:5.
Downie L, Halliday J, Lewis S, Amor DJ. Principles of genomic newborn screening programs: a systematic review. JAMA Netw Open. 2021;4:e2114336.
Holm IA, Agrawal PB, Ceyhan-Birsoy O, Christensen KD, Fayer S, Frankel LA, et al. The BabySeq project: implementing genomic sequencing in newborns. BMC Pediatr. 2018;18:225.
Genetti CA, Schwartz TS, Robinson JO, VanNoy GE, Petersen D, Pereira S, et al. Parental interest in genomic sequencing of newborns: enrollment experience from the BabySeq Project. Genet Med. 2019;21:622–30.
Ceyhan-Birsoy O, Murry JB, Machini K, Lebo MS, Yu TW, Fayer S, et al. Interpretation of genomic sequencing results in healthy and Ill newborns: results from the BabySeq project. Am J Hum Genet. 2019;104:76–93.
Pereira S, Smith HS, Frankel LA, Christensen KD, Islam R, Robinson JO, et al. Psychosocial effect of newborn genomic sequencing on families in the babyseq project: a randomized clinical trial. JAMA Pediatr. 2021;175:1132–41.
The GUARDIAN Study. What is the GUARDIAN study? 2021. https://guardian-study.org/.
Genomics England. Newborn genomes programme. 2023. https://www.genomicsengland.co.uk/initiatives/newborns.
Knowles JK, Helbig I, Metcalf CS, Lubbers LS, Isom LL, Demarest S, et al. Precision medicine for genetic epilepsy on the horizon: Recent advances, present challenges, and suggestions for continued progress. Epilepsia. 2022;63:2461–75.
Finkel RS, Mercuri E, Darras BT, Connolly AM, Kuntz NL, Kirschner J, et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377:1723–32.
Prakash V. Spinraza-a rare disease success story. Gene Ther. 2017;24:497.
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381:1644–52.
Kulkarni JA, Witzigmann D, Thomson SB, Chen S, Leavitt BR, Cullis PR, et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16:630–43.
Elliott AM, du Souich C, Lehman A, Guella I, Evans DM, Candido T, et al. RAPIDOMICS: rapid genome-wide sequencing in a neonatal intensive care unit-successes and challenges. Eur J Pediatr. 2019;178:1207–18.
Freed AS, Clowes Candadai SV, Sikes MC, Thies J, Byers HM, Dines JN, et al. The impact of rapid exome sequencing on medical management of critically Ill children. J Pediatr. 2020;226:202–12.
Smith HS, Swint JM, Lalani SR, de Oliveira Otto MC, Yamal JM, Russell HV, et al. Exome sequencing compared with standard genetic tests for critically ill infants with suspected genetic conditions. Genet Med. 2020;22:1303–10.
Hayeems RZ, Luca S, Assamad D, Bhatt A, Ungar WJ. Utility of genetic testing from the perspective of parents/caregivers: a scoping review. Children. 2021;8:259.
Kohler JN, Turbitt E, Biesecker BB. Personal utility in genomic testing: a systematic literature review. Eur J Hum Genet. 2017;25:662–8.
Callahan KP, Mueller R, Flibotte J, Largent EA, Feudtner C. Measures of utility among studies of genomic medicine for critically Ill infants: a systematic review. JAMA Netw Open. 2022;5:e2225980.
Smith HS. Genomic medicine’s critical outcome measure-utility. JAMA Netw Open. 2022;5:e2225988.
Diaby V, Babcock A, Huang Y, Moussa RK, Espinal PS, Janvier M, et al. Real-world economic evaluation of prospective rapid whole-genome sequencing compared to a matched retrospective cohort of critically ill pediatric patients in the United States. Pharmacogenomics J. 2022;22:223–9.
D’Gama AM, Agrawal PB. Role of genomic medicine and implementing equitable access for critically ill infants in neonatal intensive care units. J Perinatol. 2023;43:963–7.
Wojcik MH, Del Rosario MC, Agrawal PB. Perspectives of United States neonatologists on genetic testing practices. Genet Med. 2022;24:1372–7.
Wojcik MH, Callahan KP, Antoniou A, Del Rosario MC, Brunelli L, ElHassan NO, et al. Provision and availability of genomic medicine services in Level IV neonatal intensive care units. Genet Med. 2023;25:100926.
Douglas MP, Parker SL, Trosman JR, Slavotinek AM, Phillips KA. Private payer coverage policies for exome sequencing (ES) in pediatric patients: trends over time and analysis of evidence cited. Genet Med. 2019;21:152–60.
Trosman JR, Weldon CB, Slavotinek A, Norton ME, Douglas MP, Phillips KA. Perspectives of US private payers on insurance coverage for pediatric and prenatal exome sequencing: Results of a study from the Program in Prenatal and Pediatric Genomic Sequencing (P3EGS). Genet Med. 2020;22:283–91.
Best S, Brown H, Lunke S, Patel C, Pinner J, Barnett CP, et al. Learning from scaling up ultra-rapid genomic testing for critically ill children to a national level. NPJ Genom Med. 2021;6:5.
Stark Z, Lunke S, Brett GR, Tan NB, Stapleton R, Kumble S, et al. Meeting the challenges of implementing rapid genomic testing in acute pediatric care. Genet Med. 2018;20:1554–63.
Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50.
Franck LS, Kriz RM, Rego S, Garman K, Hobbs C, Dimmock D. Implementing rapid whole-genome sequencing in critical care: a qualitative study of facilitators and barriers to new technology adoption. J Pediatr. 2021;237:237–43.e2.
Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A’Court C, et al. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017;19:e367.
Bupp CP, Ames EG, Arenchild MK, Caylor S, Dimmock DP, Fakhoury JD, et al. Breaking barriers to rapid whole genome sequencing in pediatrics: Michigan’s Project Baby Deer. Children. 2023;10:106.
Lewis C, Buchannan J, Clarke A, Clement E, Friedrich B, Hastings-Ward J, et al. Mixed-methods evaluation of the NHS Genomic Medicine Service for paediatric rare diseases: study protocol. NIHR Open Res. 2021;1:23.
Funding
AMD was supported by NICHD/NIH (T32 HD 098061). PBA is supported by NHGRI/NIH (R01HG011798) and “Because of Bella” foundation.
Author information
Authors and Affiliations
Contributions
AMD and PBA conceived the manuscript, AMD drafted the manuscript, and AMD and PBA critically reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
D’Gama, A.M., Agrawal, P.B. Genomic medicine in neonatal care: progress and challenges. Eur J Hum Genet 31, 1357–1363 (2023). https://doi.org/10.1038/s41431-023-01464-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41431-023-01464-z
This article is cited by
-
Ambivalence and regret in genome sequencing
European Journal of Human Genetics (2023)