Abstract
Objectives
To determine the incidence and risk factors of hearing loss (HL) in Brazilian neonates.
Study design
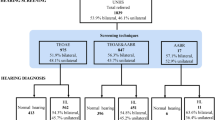
11,900 neonates were screened for hearing and congenital CMV (cCMV). Low and high-risk babies who did not pass their hearing screening and infants with cCMV were scheduled for a diagnostic audiologic evaluation.
Results
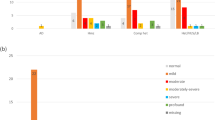
The incidence of HL was 2 per 1000 live-born infants (95% CI: 1–3). HL was higher in high-risk neonates than in low risk babies (18.6 vs. 0.3/1000 live births, respectively). Among infants exposed to isolated risk factors, association of HL with craniofacial abnormalities/syndromes (RR = 24.47; 95% CI: 5.9–100.9) and cCMV (RR = 9.54; 95% CI: 3.3–27.7) were observed. HL was 20 to 100-fold more likely in neonates exposed to ototoxic drugs in combination with cCMV or craniofacial/congenital anomalies.
Conclusions
Strategies for the prevention of cCMV and exposure to ototoxic drugs may decrease the incidence of HL in this population.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nikolopoulos TP. Neonatal hearing screening: what we have achieved and what needs to be improved. Int J Pediatr Otorhinolaryngol. 2015;79:635–7.
Meyer C, Witte J, Hildmann A, Hennecke KH, Schunck KU, Maul K, et al. Neonatal screening for hearing disorders in infants at risk: incidence, risk factors, and follow-up. Pediatrics. 1999;104:900–4.
Bielecki I, Horbulewicz A, Wolan T. Risk factors associated with hearing loss in infants: an analysis of 5282 referred neonates. Int J Pediatr Otorhinolaryngol. 2011;75:925–30.
Wróbel MJ, Greczka G, Szyfter W. The risk factor profile of children covered by the Polish universal neonatal hearing screening program and its impact on hearing loss incidence. Int J Pediatr Otorhinolaryngol. 2014;78:209–13.
Wroblewska-Seniuk K, Dabrowski P, Greczka G, Szabatowska K, Glowacka A, Szyfter W, et al. Sensorineural and conductive hearing loss in infants diagnosed in the program of universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2018;105:181–6.
Vashistha I, Aseri Y, Singh BK, Verma PC. Prevalence of hearing impairment in high risk infants. Indian J Otolaryngol Head Neck Surg. 2016;68:214–7.
Chapchap MJ, Segre CM. Universal newborn hearing screening and transient evoked otoacoustic emission: new concepts in Brazil. Scand Audio Suppl. 2001;1:33–6.
Bevilacqua MC, Alvarenga KF, Costa OA, Moret ALM. The universal newborn hearing screening in Brazil: from identification to intervention. Int J Pediatr Otorhinolaryngol. 2010;74:510–5.
Barboza ACS, Resende LM, Ferreira DBC, Lapertosa CZ, Carvalho SAS. Correlation between hearing loss and risk indicators in a neonatal hearing screening reference service. Audiol Commun Res. 2013;18:285–92. https://doi.org/10.1590/S2317-64312013000400009.
Pereira T, Costa KC, Pomilio MCA, Costa SMS, Rodrigues GRI, Sartorato EL. Etiological investigation of deaf in neonates screened in a universal newborn hearing screening program. Rev CEFAC. 2014;16:422–9.
Yamamoto AY, Anastasio ART, Tanaka EM, Isaac ML, Manfredi AKS, Cavalcante JMS, et al. Contribution of congenital cytomegalovirus (cCMV) to permanent hearing loss in a highly seropositive population: the BraCHS study. Clin Infect Dis. 2020;70:1379–84.
Yamamoto AY, Castellucci RA, Aragon DC, Mussi-Pinhata MM. Early high CMV seroprevalence in pregnant women from a population with a high rate of congenital infection. Epidemiol Infect. 2013;141:2187–91.
American Academy of Pediatrics, Joint Committee on Infant Hearing. Position statement: principles and guidelines for early hearing detection and intervention programs. Pediatrics. 2007;120:898–921.
American Academy of Pediatrics, Joint Committee on Infant Hearing. Position statement: principles and guidelines for early hearing detection and intervention programs. J Early Hear Detection Intervention. 2019;4:1–43.
Yamamoto AY, Mussi-Pinhata MM, Marin LJ, Brito RM, Oliveira PF, Coelho TB. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol. 2006;36:228–30.
British Columbia Early Hearing Program (BCEHP). A service of BC Children´s Hospital and the Provincial Health Services Authority- Audiology Assessment Protocol. 2012. Version 4.1. http://www.phsa.ca/Documents/bcehpaudiologyassessmentprotocol.pdf (Accessed 25 Feb 2019).
Stapells DR, Gravel JS, Martin BA. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear. 1995;16:361–71.
WHO. World Health Organization. Prevention of blindness and deafness. Grades of hearing impairment. http://who.int/pbd/deafness/hearing_impairment_grades/en (Accessed 25 Feb 2019).
Speybroeck N. Classification and regression trees. Int J Public Health. 2012;57:243–6.
Ohl C, Dornier L, Czajka C, Chobault JC, Tavernier L. Newborn hearing screening on infants at risk. Int J Pediatr Otorhinolaryngol. 2009;73:1691–5.
Wroblewska-Seniuk K, Greczka G, Dabrowski P, Szyfter W, Mazela J. The results of newborn hearing screening by means of transient otoacoustic emissions - has anything changed over 10 years? Int J Pediatr Otorhinolaryngol. 2017;96:4–10.
Johnson JL, White KR, Widen JE, Gravel JS, James M, Kennalley T, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116:663–72.
Levit Y, Himmelfarb M, Dollber S. Sensitivity of the automated auditory brainstem response in neonatal hearing screening. Pediatrics. 2015;136:641–7.
Saki N, Bayat A, Hoseinabadi R, Nikakhlagh S, Karimi M, Dashti R. Universal newborn hearing screening in southwestern Iran. Int J Pediatr Otorhinolaryngol. 2017;97:89–92.
White KR, Vohr BR, Maxon AB. Screening all newborns for hearing loss using transient evoked otoacoustic emissions. Int J Pediatr Otorhinolaryngol. 1994;29:203–17.
Barsky-Firkser L, Sun S. Universal newborn hearing screenings: a three-year experience. Pediatrics. 1997;99:E4.
Finitzo T, Albright K, O’Neal J. The newborn with hearing loss: detection in the nursery. Pediatrics. 1998;102:1452–60.
Ngo RY, Tan HK, Balakrishnan A, Lim SB, Lazaroo DT. Auditory neuropathy/auditory dys-synchrony detected by universal newborn hearing screening. Int J Pediatr Otorhinolaryngol. 2006;70:1299–306.
Boudewyns A, Declau F, Van Den Ende J, Dirckx S, Van de Heyning P. Auditory neuropathy spectrum disorder (ANSD) in referrals from neonatal hearing screening at a well-baby clinic. Eur J Pediatr. 2016;175:993–1000.
Cone-Wesson B, Vohr BR, Sininger YS, Widen JE, Folsom RC, Gorga MP, et al. Identification of neonatal hearing impairment: infants with hearing loss. Ear Hear. 2000;21:488–507.
Dumanch KA, Holte L, O’Hollearn T, Walker E, Clark J, Oleson J. High risk factors associated with early childhood hearing loss: a 3-year review. Am J Audio. 2017;26:129–42.
Onoda RM, Azevedo MF, Santos AM. Neonatal hearing screening: failures, hearing loss and risk indicators. Braz J Otorhinolaryngol. 2011;77:775–83.
Fuchs A, Zimmermann L, Graz MB, Cherpillod J, Tolsa JF, Buclin T, et al. Gentamicin exposure and sensorineural hearing loss in preterm infants. PLoS ONE. 2016;8:1–11.
Puia-Dumitrescu M, Bretzius OM, Brown N, Fitz-Henley JA, Ssengonzi R, Wechsler CS, et al. Evaluation of gentamicin exposure in the neonatal intensive care unit and hearing function at discharge. J Pediatr. 2018;203:131–6.
Funding
All phases of this study were supported by FAPESP, Brazil, (Grant 2013/06579-0 to MMMP); and Eunice Kennedy Shriver NICHD, USA (Grant 2R01HD061959-07A2 to WJB).
Author information
Authors and Affiliations
Contributions
ARTA conceptualized and designed the study, drafted the initial manuscript, revised critically and approved the final version of the manuscript. She also supervised the application of the hearing screening and audiological tests. AYY conceptualized and designed the study, supervised the CMV screening and the follow up of CMV-infected infants, was in charge of data management, revised critically and approved the final version of the manuscript. ETM conceptualized and designed the study, provided supervision and care for infants with suspected and confirmed hearing loss, helped with data acquisition, revised critically and approved the final version of the manuscript. MM M-P conceptualized the BRACHS cohort study, obtained funds, supervised activities for data collection, took part of the manuscript preparation, and definition of data analysis, revised critically and approved the final version of the manuscript. AKSM and JMSC participated in the application of the hearing screening and audiological tests, being in charge of the completeness of the audiological protocol activities and data acquisition, revised critically and approved the final version of the manuscript. BCPL participated in the care of the children who underwent electrophysiological examinations under anesthesia, helped with data acquisition, revised critically and approved the final version of the manuscript. DCA carried out the statistical analyses and interpretation of data, revised critically and approved the final version of the manuscript. SB conceptualized the BRACHS cohort study, and reviewed the manuscript for important intellectual content and approved the final version of the manuscript. KBF participated in the conceptualization and data managing planning for the BRACHS cohort study, reviewed and approved the final version of the manuscript. WJB conceptualized the BRACHS cohort study, and reviewed the manuscript for important intellectual content, and approved the final version of the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects related to the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Anastasio, A.R.T., Yamamoto, A.Y., Massuda, E.T. et al. Comprehensive evaluation of risk factors for neonatal hearing loss in a large Brazilian cohort. J Perinatol 41, 315–323 (2021). https://doi.org/10.1038/s41372-020-00807-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41372-020-00807-8
This article is cited by
-
Risk factors for infant hearing loss: a meta-analysis
European Journal of Pediatrics (2024)
-
Sheep as a large animal model for hearing research: comparison to common laboratory animals and humans
Laboratory Animal Research (2023)
-
Genetic etiology of non-syndromic hearing loss in Latin America
Human Genetics (2022)