Abstract
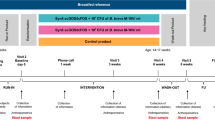
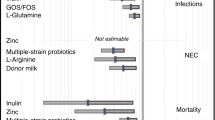
Postbiotics, as emerging products, were added to infant formula, but their safety and efficacy are unclear. To clarify this issue, we wrote this meta-analysis. We searched PubMed, Embase, Web of Science and ProQuest from its establishment to February 2023. The review was registered on PROSPERO database (CRD42022352405). The effects of infant formula with and without postbiotics were compared, and the incidence of serious adverse events (SAEs), digestive symptoms, concentration of stool secretory immunoglobulin A (SIgA), and growth and development indexes were analyzed. Nine randomized controlled trials with 2065 participants were included. The addition of postbiotics to infant formula was found to increase the concentration of stool SIgA (P < 0.05) with very low certainty of evidence, without significantly impacting the incidence of SAEs, infantile colic, flatulence, diarrhea, vomiting, abdominal pain and gastrointestinal disorders, the daily weight gain, the total gain in body length and the daily head circumference gain (all P > 0.05). Adding postbiotics to the formula is safe for infants, which would not increase the incidence of SAEs, infantile colic, flatulence, diarrhea, vomiting, abdominal pain, and gastrointestinal disorders, and could increase the concentration of stool SIgA.
Impact
-
Our study provides evidence that the addition of postbiotics to infant formula is safe but not effective.
-
This is the first systematic review and meta-analysis of postbiotics.
-
This study provides strong evidence for the safety of postbiotics and lays a foundation for related clinical trials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 14 print issues and online access
$259.00 per year
only $18.50 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Salminen, S. et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 18, 649–667 (2021).
Gao, J. et al. Probiotics in the dairy industry-advances and opportunities. Compr. Rev. Food Sci. Food Saf. 20, 3937–3982 (2021).
Piqué, N., Berlanga, M. & Miñana-Galbis, D. Health benefits of heat-killed (tyndallized) probiotics: an overview. Int. J. Mol. Sci. 20, 2534 (2019).
Malagón-Rojas, J. N., Mantziari, A., Salminen, S. & Szajewska, H. Postbiotics for preventing and treating common infectious diseases in children: a systematic review. Nutrients 12, 389 (2020).
Chuah, L. O. et al. Postbiotic metabolites produced by lactobacillus plantarum strains exert selective cytotoxicity effects on cancer cells. BMC Complement. Altern. Med. 19, 114 (2019).
Kareem, K. Y., Loh, T. C., Foo, H. L., Asmara, S. A. & Akit, H. Influence of Postbiotic Rg14 and inulin combination on cecal microbiota, organic acid concentration, and cytokine expression in broiler chickens. Poult. Sci. 96, 966–975 (2017).
Wegh, C. A. M., Geerlings, S. Y., Knol, J., Roeselers, G. & Belzer, C. Postbiotics and their potential applications in early life nutrition and beyond. Int. J. Mol. Sci. 20, 4673 (2019).
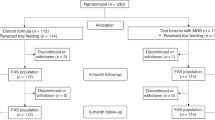
Moher, D., Liberati, A., Tetzlaff, J. & Altman, D. G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 6, e1000097 (2009).
Rodriguez-Herrera, A. et al. Gastrointestinal tolerance, growth and safety of a partly fermented formula with specific prebiotics in healthy infants: a double-blind, randomized, controlled trial. Nutrients 11, 1530 (2019).
Thibault, H., Aubert-Jacquin, C. & Goulet, O. Effects of long-term consumption of a fermented infant formula (with Bifidobacterium Breve C50 and Streptococcus Thermophilus 065) on acute diarrhea in healthy infants. J. Pediatr. Gastroenterol. Nutr. 39, 147–152 (2004).
Vandenplas, Y. et al. A partly fermented infant formula with postbiotics including 3’-Gl, specific oligosaccharides, 2’-Fl, and milk fat supports adequate growth, is safe and well-tolerated in healthy term infants: a double-blind, randomised, controlled, multi-country trial. Nutrients 12, 3560 (2020).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 5, 13 (2005).
Campeotto, F. et al. A fermented formula in pre-term infants: clinical tolerance, gut microbiota, down-regulation of faecal calprotectin and up-regulation of faecal secretory IgA. Br. J. Nutr. 105, 1843–1851 (2011).
Huet, F. et al. Partly fermented infant formulae with specific oligosaccharides support adequate infant growth and are well-tolerated. J. Pediatr. Gastroenterol. Nutr. 63, e43–e53 (2016).
Mensah, P. et al. Feeding of lactic acid-fermented high nutrient density weaning formula in paediatric settings in Ghana and Nigeria: acceptance by mother and infant and performance during recovery from acute diarrhoea. Int. J. Food Sci. Nutr. 46, 353–362 (1995).
Bellaiche, M. et al. Safety and tolerance of a novel anti-regurgitation formula: a double-blind, randomized, controlled trial. J. Pediatr. Gastroenterol. Nutr. 73, 579–585 (2021).
Morisset, M., Aubert-Jacquin, C., Soulaines, P., Moneret-Vautrin, D. A. & Dupont, C. A non-hydrolyzed, fermented milk formula reduces digestive and respiratory events in infants at high risk of allergy. Eur. J. Clin. Nutr. 65, 175–183 (2011).
Rodriguez-Herrera, A. et al. Early-life fecal microbiome and metabolome dynamics in response to an intervention with infant formula containing specific prebiotics and postbiotics. Am. J. Physiol. Gastrointest. Liver Physiol. 322, G571–g582 (2022).
Plaza-Diaz, J. et al. Effects of a novel infant formula on weight gain, body composition, safety and tolerability to infants: The Innova 2020 Study. Nutrients 15, 147 (2022).
Roggero, P. et al. Analysis of immune, microbiota and metabolome maturation in infants in a clinical trial of Lactobacillus paracasei Cba l74-fermented formula. Nat. Commun. 11, 2703 (2020).
Béghin, L. et al. Fermented infant formula (with Bifidobacterium Breve C50 and Streptococcus Thermophilus O65) with prebiotic oligosaccharides is safe and modulates the gut microbiota towards a microbiota closer to that of breastfed infants. Clin. Nutr. 40, 778–787 (2021).
Vandenplas, Y. et al. Randomised controlled trial demonstrates that fermented infant formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides reduces the incidence of infantile colic. Acta Paediatr. 106, 1150–1158 (2017).
Szajewska, H. & Salminen, S. Evidence on postbiotics in infants and children. Curr. Opin. Clin. Nutr. Metab. Care 26, 253–258 (2023).
Liang, B. & Xing, D. The current and future perspectives of postbiotics. Probiotics Antimicrob. Proteins 1–18 https://doi.org/10.1007/s12602-023-10045-x (2023).
Ma, L., Tu, H. & Chen, T. Postbiotics in human health: a narrative review. Nutrients 15, 291 (2023).
Szydłowska, A. & Sionek, B. Probiotics and postbiotics as the functional food components affecting the immune response. Microorganisms 11, 104 (2022).
Hidalgo-Cantabrana, C. et al. Bifidobacteria and their health-promoting effects. Microbiol Spectr. 5, https://doi.org/10.1128/microbiolspec.BAD-0010-2016 (2017).
Park, I. S. et al. Bifidobacterium Breve Cbt Br3 is effective at relieving intestinal inflammation by augmenting goblet cell regeneration. J. Gastroenterol. Hepatol. 38, 1346–1354 (2023).
Wu, T. et al. Comparison of volatile metabolic profiles in fermented milk of Streptococcus thermophilus during the postripening period at different incubation temperatures. J. Dairy Sci. 106, 2303–2313 (2023).
Niu, M. M., Guo, H. X., Cai, J. W. & Meng, X. C. Bifidobacterium Breve alleviates DSS-induced colitis in mice by maintaining the mucosal and epithelial barriers and modulating gut microbes. Nutrients 14, 3671 (2022).
Tian, P. et al. Bifidobacterium Breve Ccfm1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav. Immun. 100, 233–241 (2022).
Wang, Q. et al. Bifidobacterium Breve and bifidobacterium longum attenuate choline-induced plasma trimethylamine N-oxide production by modulating gut microbiota in mice. Nutrients 14, 1222 (2022).
Savary-Auzeloux, I. et al. Anti-inflammatory Streptococcus thermophilus Cnrz160 limits sarcopenia induced by low-grade inflammation in older adult rats. Front. Nutr. 9, 986542 (2022).
Daelemans, S., Peeters, L., Hauser, B. & Vandenplas, Y. Recent advances in understanding and managing infantile colic. F1000Res. 7, F1000 (2018).
Martinelli, M. et al. Efficacy of a standardized extract of matricariae Chamomilla L., Melissa Officinalis L. and Tyndallized Lactobacillus Acidophilus (Ha122) in infantile colic: an open randomized controlled trial. Neurogastroenterol Motil. 29, https://doi.org/10.1111/nmo.13145 2017.
Chauvière, G. et al. Competitive exclusion of diarrheagenic Escherichia Coli (Etec) from human enterocyte-like Caco-2 Cells by heat-killed lactobacillus. FEMS Microbiol. Lett. 70, 213–217 (1992).
Nataraj, B. H., Ali, S. A., Behare, P. V. & Yadav, H. Postbiotics-parabiotics: the new horizons in microbial biotherapy and functional foods. Microb. Cell Fact. 19, 168 (2020).
Rognum, T. O., Thrane, S., Stoltenberg, L., Vege, A. & Brandtzaeg, P. Development of intestinal mucosal immunity in fetal life and the first postnatal months. Pediatr. Res. 32, 145–149 (1992).
Corthésy, B. Multi-faceted functions of secretory IgA at mucosal surfaces. Front. Immunol. 4, 185 (2013).
Ding, M. et al. Crosstalk between Siga-coated bacteria in infant gut and early-life health. Trends Microbiol. 29, 725–735 (2021).
Mahdally, S. M. et al. Secretory-IgA binding to intestinal microbiota attenuates inflammatory reactions as the intestinal barrier of preterm infants matures. Clin. Exp. Immunol. https://doi.org/10.1093/cei/uxad042 (2023).
Gopalakrishna, K. P. & Hand, T. W. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients 12, 823 (2020).
de Fays, C., Carlier, F. M., Gohy, S. & Pilette, C. Secretory immunoglobulin a immunity in chronic obstructive respiratory diseases. Cells 11, 1324 (2022).
Author information
Authors and Affiliations
Contributions
J.S. and C.C. designed the study and reviewed the manuscript. X.L. drafted the manuscript. X.L. and C.C. screened the literature. X.L., Y.L., Z.Z. and R.D. extracted and analyzed the data. J.S. and C.C. revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, X., Li, Y., Zhao, Z. et al. Safety and efficacy of adding postbiotics in infant formula: a systematic review and meta-analysis. Pediatr Res 95, 43–51 (2024). https://doi.org/10.1038/s41390-023-02813-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-023-02813-w