Abstract
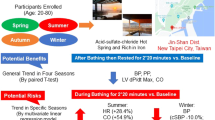
Emerging evidence suggests beneficial effects of sauna bathing on the cardiovascular system. However, the effects of sauna bathing on parameters of cardiovascular function and blood-based biomarkers are uncertain. We aimed to investigate whether sauna bathing induces changes in arterial stiffness, blood pressure (BP), and several blood-based biomarkers. We conducted an experimental study including 102 participants (mean age (SD): 51.9 (9.2) years, 56% male) who had at least one cardiovascular risk factor. Participants were exposed to a single sauna session (duration: 30 min; temperature: 73 °C; humidity: 10–20%). Cardiovascular as well as blood-based parameters were collected before, immediately after, and after 30-min recovery. Mean carotid–femoral pulse wave velocity was 9.8 (2.4) m/s before sauna and decreased to 8.6 (1.6) m/s immediately after sauna (p < 0.0001). Mean systolic BP decreased after sauna exposure from 137 (16) to 130 (14) mmHg (p < 0.0001) and diastolic BP from 82 (10) to 75 (9) mmHg (p < 0.0001). Systolic BP after 30 min recovery remained lower compared to pre-sauna levels. There were significant changes in hematological variables during sauna bathing. Plasma creatinine levels increased slightly from sauna until recovery period, whereas sodium and potassium levels remained constant. This study demonstrates that sauna bathing for 30 min has beneficial effects on arterial stiffness, BP, and some blood-based biomarkers. These findings may provide new insights underlying the emerging associations between sauna bathing and reduced risk of cardiovascular outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Perasalo J. Traditional use of the sauna for hygiene and health in Finland. Ann Clin Res. 1988;20:220–3.
Valtakari P. The sauna and bathing in different countries. Ann Clin Res. 1988;20:230–5.
Ohori T, Nozawa T, Ihori H, Shida T, Sobajima M, Matsuki A, et al. Effect of repeated sauna treatment on exercise tolerance and endothelial function in patients with chronic heart failure. Am J Cardiol. 2012;109:100–4.
Leppäluoto J, Tuominen M, Väänänen A, Karpakka J, Vuori J. Some cardiovascular and metabolic effects of repeated sauna bathing. Acta Physiol Scand. 1986;128:77–81.
Brunt VE, Eymann TM, Francisco MA, Howard MJ, Minson CT. Passive heat therapy improves cutaneous microvascular function in sedentary humans via improved nitric oxide-dependent dilation. J Appl Physiol. 2016;121:716–23.
Brunt VE, Howard MJ, Francisco MA, Ely BR, Minson CT. Passive heat therapy improves endothelial function, arterial stiffness and blood pressure in sedentary humans. J Physiol. 2016;594:5329–42.
Imamura M, Biro S, Kihara T, Yoshifuku S, Takasaki K, Otsuji Y, et al. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J Am Coll Cardiol. 2001;38:1083–8.
Giannetti N, Juneau M, Arsenault A, Behr MA, Grégoire J, Tessier M, et al. Sauna-induced myocardial ischemia in patients with coronary artery disease. Am J Med. 1999;107:228–33.
Gayda M, Paillard F, Sosner P, Juneau M, Garzon M, Gonzalez M, et al. Effects of sauna alone and postexercise sauna baths on blood pressure and hemodynamic variables in patients with untreated hypertension. J Clin Hypertens. 2012;14:553–60.
Kunutsor S. Ambient temperature or seasonal variations in blood pressure: how important is this in sub-Saharan Africa? Ethn Dis. 2010;20:1.
Kunutsor SK, Powles JW. The effect of ambient temperature on blood pressure in a rural West African adult population: a cross-sectional study. Cardiovasc J Afr. 2010;21:17–20.
Laukkanen T, Khan H, Zaccardi F, Laukkanen JA. Association between sauna bathing and fatal cardiovascular and all-cause mortality events. JAMA Intern Med. 2015;175:542–8.
Zaccardi F, Laukkanen T, Willeit P, Kunutsor SK, Kauhanen J, Laukkanen JA. Sauna bathing and incident hypertension: a prospective cohort study. Am J Hypertens. 2017;30:1120–5.
Laukkanen T, Kunutsor S, Kauhanen J, Laukkanen JA. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing. 2017;46:245–9.
Kukkonen-Harjula K, Kauppinen K. Health effects and risks of sauna bathing. Int J Circumpolar Health. 2006;65:195–205.
Hannuksela ML, Ellahham S. Benefits and risks of sauna bathing. Am J Med. 2001;110:118–26.
Crandall CG, González-Alonso J. Cardiovascular function in the heat-stressed human. Acta Physiol. 2010;199:407–23.
Hillebrand S, Gast KB, de Mutsert R, Swenne CA, Jukema JW, Middeldorp S, et al. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: meta-analysis and dose-response meta-regression. Europace. 2013;15:742–9.
Lietava J, Vohnout B, Valent D, Celko J. Comparison of hemodynamics during hyperthermal immersion and exercise testing in apparently healthy females aged 50-60 years. Ital Heart J. 2004;5:511–6.
Stanley J, Halliday A, D’Auria S, Buchheit M, Leicht AS. Effect of sauna-based heat acclimation on plasma volume and heart rate variability. Eur J Appl Physiol. 2015;115:785–94.
Salonen JT. Is there a continuing need for longitudinal epidemiologic research? The Kuopio Ischaemic Heart Disease Risk Factor Study. Ann Clin Res. 1988;20:46–50.
Laukkanen JA, Laaksonen D, Lakka TA, Savonen K, Rauramaa R, Mäkikallio T, et al. Determinants of cardiorespiratory fitness in men aged 42 to 60 years with and without cardiovascular disease. Am J Cardiol. 2009;103:1598–604.
Lakka TA, Venäläinen JM, Rauramaa R, Salonen R, Tuomilehto J, Salonen JT. Relation of leisure-time physical activity and cardiorespiratory fitness to the risk of acute myocardial infarction. N Engl J Med. 1994;330:1549–54.
Tomlinson LA. Methods for assessing arterial stiffness: technical considerations. Curr Opin Nephrol Hypertens. 2012;21:655–60.
Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8.
Wiesel J, Arbesfeld B, Schechter D. Comparison of the Microlife blood pressure monitor with the Omron blood pressure monitor for detecting atrial fibrillation. Am J Cardiol. 2014;114:1046–8.
Salvi P, Lio G, Labat C, Ricci E, Pannier B, Benetos A. Validation of a new non-invasive portable tonometer for determining arterial pressure wave and pulse wave velocity: the PulsePen device. J Hypertens. 2004;22:2285–93.
Joly L, Perret-Guillaume C, Kearney-Schwartz A, Salvi P, Mandry D, Marie PY, et al. Pulse wave velocity assessment by external noninvasive devices and phase-contrast magnetic resonance imaging in the obese. Hypertension. 2009;54:421–6.
Salvi P, Palombo C, Salvi GM, Labat C, Parati G, Benetos A. Left ventricular ejection time, not heart rate, is an independent correlate of aortic pulse wave velocity. J Appl Physiol. 2013;115:1610–7.
Redberg RF. Health benefits of sauna bathing. JAMA Intern Med. 2015;175:548.
Thomas KN, van Rij AM, Lucas SJ, Cotter JD. Lower-limb hot-water immersion acutely induces beneficial hemodynamic and cardiovascular responses in peripheral arterial disease and healthy, elderly controls. Am J Physiol Regul Integr Comp Physiol. 2017;312:R281–91.
Franklin SS. Beyond blood pressure: arterial stiffness as a new biomarker of cardiovascular disease. J Am Soc Hypertens. 2008;2:140–51.
Schoffstall JE, Branch JD, Leutholtz BC, Swain DE. Effects of dehydration and rehydration on the one-repetition maximum bench press of weight-trained males. J Strength Cond Res. 2001;15:102–8.
Acknowledgments
We sincerely thank Timo Harvia and the staff of Harvia Oy for sauna test facilities of the research and the subjects for their dedicated participation in the study.
Fundings
This study was supported by the Tekes, the Finnish Funding Agency for Technology and Innovation, Helsinki, Finland. Collaborators of the study project were Harvia Oy, Velha Oy, Pihlajalinna Clinic, Fintravel Oy, Finnish Sauna Culture Society and University of Eastern Finland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Laukkanen, T., Kunutsor, S.K., Zaccardi, F. et al. Acute effects of sauna bathing on cardiovascular function. J Hum Hypertens 32, 129–138 (2018). https://doi.org/10.1038/s41371-017-0008-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41371-017-0008-z
This article is cited by
-
Synchronized wearables for the detection of haemodynamic states via electrocardiography and multispectral photoplethysmography
Nature Biomedical Engineering (2023)
-
Athlete and practitioner prevalence, practices, and perceptions of passive heating in sport
Sport Sciences for Health (2023)
-
The Interplay between Systolic Blood Pressure, Sauna Bathing, and Cardiovascular Mortality in Middle-Aged and Older Finnish Men: A Cohort Study
The Journal of nutrition, health and aging (2023)
-
Inflammation, sauna bathing, and all-cause mortality in middle-aged and older Finnish men: a cohort study
European Journal of Epidemiology (2022)
-
Serum copper-to-zinc ratio and risk of incident pneumonia in caucasian men: a prospective cohort study
BioMetals (2022)