Abstract
Hot water bathing has been demonstrated to be an effective way to improve people’s cardiovascular health in many studies. This study focused on seasonal physiological changes to provide suggestions on bathing methods based on season for hot spring bathing. Volunteers were recruited to the program of hot spring bathing at 38–40 °C in New Taipei City. Cardiovascular function, blood oxygen, and ear temperature were observed. There were five assessments for each participant during the study process: baseline, bathing for 20 min and 2 cycles *20 (2*20) min, resting for 20 min and 2*20 min after bathing, respectively. Lower blood pressure (p < 0.001), pulse pressure (p < 0.001), left ventricular dP/dt Max (p < 0.001), and cardiac output (p < 0.05) were identified after bathing then rested for 2*20 min in four seasons, compared to baseline by paired T test. However, in multivariate linear regression model, potential risk for bathing in summer was assumed by higher heart rate (+28.4%, p < 0.001), cardiac output (+54.9%, p < 0.001) and left ventricular dP/dt Max (+27.6%, p < 0.05) during bathing at 2*20 min in summer. Potential risk for bathing in winter was postulated by blood pressure lowering (cSBP −10.0%; cDBP −22.1%, p < 0.001) during bathing at 2*20 min in winter. Hot spring bathing is shown to potentially improve cardiovascular function via reducing cardiac workload and vasodilation effects. Prolonged hot spring bathing in summer is not suggested due to significantly increased cardiac stress. In winter, prominent drop of blood pressure should be concerned.

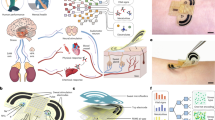
We demonstrated the study enrollment, the hot-spring contents and location, and physiological changes of general trends or seasonal variations, which may indicate potential benefits and risks during and after bathing. (Abbreviations: BP, blood pressure; PP, pulse pressure; LV, left ventricular; CO, cardiac output; HR, heart rate; cSBP, central systolic blood pressure; cDBP, central diastolic blood pressure).
Similar content being viewed by others
Introduction
Balneotherapy is a way for cardiovascular health promotion, which is defined as physical therapy for assisting disease treatment by bathing in hot tap water or mineral water. For several decades, balneotherapy in disease treatment effect and health promotion had been discussed worldwide, which showed significant effects for some diseases [1]. With regard to cardiovascular diseases, patients with coronary heart disease [2], hypertension [3], arrhythmia [4], and varicose vein [5] have significant improvement from balneotherapy. Therefore, balneotherapy may be a promising strategy for cardiovascular health promotion. However, balneotherapy also raises health concern especially for the elderly population. It is estimated that around 19,000 Japanese individuals die annually while taking a bath, mostly during winter, and most victims are elderly people [6, 7]. Japan had the highest number of victims from drowning and highest incidence of bathtub accidents (64.6%) among 60 countries [6, 8]. In both Japanese and Taiwanese, hot-spring bathing is part of the gathering or recreational culture, which warrants further studies on balneotherapy cardiovascular health effects.
Cardiovascular diseases (CVDs) remain the most common cause of death worldwide.More than 17 million people die from CVDs each year, this made up of 48% of non-communicable disease mortality. By 2030, the death annual toll is projected to be 23.6 million [9, 10]. Global Burden of Disease Study, a systematic analysis presented 235 causes of death and 67 different risk factors in 21 different regions worldwide. In non-transmitted diseases, cardiovascular diseases were the most prevalent, and had the highest incidence and mortality rate between 1980 and 2010 [11]. Due to high prevalence, incidence and mortality of CVDs, “appropriate balneotherapy” should be figured out for promotion of cardiovascular health and prevention from potential cardiovascular risks during hot spring bathing.
Cardiovascular physical responses, such as blood pressure, cardiac and vascular function, and heart rate variability correlated with sympathetic tone, have been associated with thermal and water immersion physical effect during hot-spring bathing is an important health issue for the safety of travelers. Jem et al. addressed heat stress effect on vascular outcomes, and many studies have demonstrated the beneficial effects of heating on reducing arterial stiffness (e.g., pulse-wave velocity and arterial wall compliance) [12]. ŞAŞ et al. recruited ninety-eight patients with musculoskeletal disorders referred to physiotherapy with balneotherapy. Diastolic blood pressure (DBP) decreased and pulse increased during balneotherapy (p < 0.05). DBP increase and pulse rate decrease were observed during recovery time (p < 0.05), which may indicate decreased pulse pressure and cardiac stress, and increased vascular elasticity compared to the status before bathing therapy. Therefore, balneotherapy may be effective for improving peripheral cardiopulmonary responses in patients with musculoskeletal disorders [13], however, the drop of blood pressure and increased cardiac stress should be concerned during balneotherapy.
Seasonal variations of cardiovascular effects during hot-spring bathing were less covered by previous studies. Accidental bathtub drowning accidents [6] and sudden death phenomenon while bathing was prominent in winter [7], however, studies on the potential threats and benefits in different seasons are limited. That is, we measured cardiovascular physiological changes in four seasons for preventive measures. Studies on changes in cardiac output, workload and blood oxygen associated with temperature changes from low to high surrounding temperature which may increases risk are also limited. Though previous studies showed peripheral blood pressure dropped during bathing, central blood pressure change has not been well studied. In addition, left ventricular (LV) work load in terms of LV dP/dt max, before and after balneotherapy has not been investigated.
This observational pilot study was designed to determine the seasonal variation in cardiovascular physiological changes and potential instant health effects in cardiovascular functions (CVFs). The aims of this study are to demonstrate seasonal variation in CVFs by comparing intra-individual differences during and after bathing in four seasons for analyzing especially on CVFs by using mixed effects model, to discuss the potential health risks and benefits of seasonal variation in cardiovascular hemodynamics during and after hot-spring bathing and to provide guidance on the bathing methods should be considered depending on the season.
Methods
Design and participants
We recruited healthy volunteers to participate in the cardiovascular promotion program of hot spring bathing and cardiovascular effects. Our study took place at Old Jinshan Governor-General Hot Spring located in New Taipei City (Graphical abstract). The hot spring water quality was analyzed three times in different seasons and was categorized as acid-sulfate-chloride hot spring and rich in iron. Hot spring temperature was maintained at about 38–40 °C during study, according to the Japanese study recommendation, which balanced between lowering heart load by reduced blood pressure and higher cardiac stress triggered by increasing heart rate under higher temperature [14, 15]. Hot spring temperature may be affected by wind speed and environmental temperature, therefore instant cold and hot water supplement was given.
The tests were performed once per month in 2019 since March for a total of seven tests. 20–80 years old adults were recruited, and past histories with acute coronary heart disease or current serious medical conditions were excluded. The number of participating subjects enrolled were 17, 24, 14, 17, 18, 13, 15, respectively. (Fig. 1) The definitions of habits shown as follows, previous and present smoking habit were both included as having smoking habit; drinking habit unless once per week defined as having alcohol habit; exercise unless three times per week, and more than 30 min each time was named as having exercise habit. 38 participants (32.2%) have hypertension. Hypertension definition included present hypertensive medication use (24 participants, 20.34%), underlying disease with hypertension and first blood pressure measurement during our tests exceeding 140/90 mmHg. 41 participants (34.75%) had hot spring bathing habit, defined as unless 12 times bathing per year, and maintained over 12 months. The data were approved by the National Taiwan University Hospital Research Ethics Committee (NTUHREC). We had well explained the study process and provided written informed consent before undergoing cardiovascular health examinations. No acute cardiovascular complications were observed during the study.
The electrocardiogram and heart rate variability data of each subject was collected throughout the whole study process. Cardiovascular function using DynaPulse were conducted five times for each participant during the study process, including baseline, 20 min and two cycles of 20 min (2*20 min) during hot spring bathing, and resting 20 min and 2*20 min after bathing, respectively (Fig. 2). According to previous Japanese studies, 15–20 min was the optimal duration for hot spring bathing [14, 15]. In addition, blood oxygen saturation level (SpO2) and ear temperature measurements were also recorded simultaneously for surrogate physiologic measures.
The protocol began with a first measurement before bathing, then the subjects were immersed in hot spring in a sitting posture to the chest level for 20 min and take the second measurement. The subjects then repeated the immersion continuously for 20 min and received the third measurement. After that, the subjects rest for 20 min and take the fourth measurement, then rest another 20 min and take the final fifth measurement. During the testing process, participants were not allowed any food intake, therefore, 300 ml of bottled water were provided. In addition, any other drinks were also restricted.
Environmental and participants’ oxygenation assessment
The instruments used for environmental monitoring were real-time recording at men’s and women’s bathing field in four different seasons. The concentration and size distribution, as well as the real-time mass concentration of particulate matter in the air (PM10, PM2.5, PM1.0, and total suspended particles), were monitored using a DustTrak aerosol monitor (model 8533; TSI Inc., Shoreview, MN, USA). The temperature, CO, CO2, and relative humidity were monitored using an IAQ monitor (model 7575; TSI Inc., Shoreview, MN, USA). Oxygen saturation rate (finger SpO2 value) was obtained using the Rossmax Pulse Oximeter, Model SB100 (Rossmax International Ltd., Taipei, Taiwan). Real-time recording of environmental parameters was saved in every minute.
Cardiac and vascular functions assessments
An oscillometric BP device (DynaPulse 200 M, Pulse Metric Inc., San Diego, CA, USA) was used to record the arterial pressure waveform using a cuff sphygmomanometer [16, 17]. BP was measured twice (left and right hands) after at least 5 min of rest in a sitting position in a quiet room. BP was determined by changes in pressure waveform according to Bernoulli flow effects. Central SBP and DBP, vascular compliance (e.g., BAC, BAD, and BAR) and peripheral resistance of the brachial artery were derived by incorporating the arterial pressure signals from a standard cuff sphygmomanometer using a physical model [18, 19]. The BP used in the analyses was compared intra-individually in left and right arm for each subject, thus two measurements for each participant. This method has also been employed to derive other cardiac hemodynamic parameters, such as the maximum rate of left ventricular pressure increase (LV dP/dt max), stroke volume (SV), CO, and cardiac index (CI); and its application was also validated in our recent studies [20,21,22]. The data were electronically transmitted from the collection site to a central analysis center.
Statistical analysis
Every participant enrolled in our study had measurements for five times before, during and after hot-spring bathing. Therefore, CVFs, blood oxygenation, ear temperature, and ambient temperature had included five data points for each measurement. For cardiovascular function monitoring, participants’ both hands were measured, then average value was recorded. Ambient temperature was monitored since the first participant’s first CVF measurement, then recorded for 20 min afterwards. Ambient temperature records in five-time measurements and the fields for men and women were monitored separately and respectively.
For evaluation of physical changes before, during and after hot spring bathing, paired T-test was used to compare the means ± standard deviations of continuous variables between baseline (before hot-spring bathing) and four sequential measurements of continuous variables such as CVFs, blood oxygenation, ear temperature, and ambient temperature. We calculated Bonferroni correction for the p-values in Table 2.
Univariate and multivariate linear regression models were applied to explore both vascular (∆SBP, ∆DBP) and cardiac effects (∆HR, ∆LV dP/dt Max, ∆CO) during hot spring bathing for two cycles of 20-min (2*20 min) and after hot spring bathing then rested for 2*20 min. Due to consideration of missing measurements, proper numbers of cardiovascular parameters were chosen to prevent overfitting. The study targeted on hot spring effect on CVFs, therefore less direct parameters such as BMI, smoking, alcohol and exercise habits were excluded in mixed effect models. Parameters included into the model were: age, gender, hypertension with medication, hot spring bathing habit, ear temperature change, ambient temperature and season. We had excluded ear and ambient temperature in multivariate regression analysis. All statistical analyses were performed with R statistical software (version 3.5.1, R Development Core Team, Vienna, Austria, 2018). Statistical significance was considered p value of < 0.05.
Hot spring content assessment
Ion contents assessment
The instruments used for hot-spring ion content assessment was Dionex ICS-1100 Ion Chromatography. (Please refer Supplementary Information 1.1 and 2.1 for detailed information.)
Microbes assessment
For detection of spring water hygiene, Escherichia coli (E. coli) and total florae were tested. (Please refer Supplementary Information 1.2 and 2.2 for detailed information.)
Results
The general characteristics of the participants are summarized in Table 1. 118 participants were enrolled. The mean ages of participants in spring, summer, autumn and winter were 59, 64, 61, and 56 years, respectively. There were 56 male participants (47.46%), and gender ratio was approximate to 1:1. When it comes to habits, there were 22 participants (18.64%) with smoking habit, 20 participants (16.95%) with alcohol habit, and 53 participants (44.92%) with exercise habit.
According to general characteristics in four seasons, no significant difference was noted between four groups in age, gender, BMI, smoking, alcohol, exercise habit, or hypertension with or without medication, hypercholesterolemia, and hot spring bathing habit (Table 1).
Table 2 presents comparisons of cardiovascular function using the DynaPulse monitoring device before, during and after hot spring bathing in the average of four seasons. During bathing for 2*20 min, vascular parameters SBP, DBP and BAD were decreased (p < 0.001), while no significant change in PP. Cardiac parameters such as HR and CO were increased (p < 0.001). Blood oxygen was decreased (p < 0.001). Ear temperature was increased (p < 0.001). After bathing and rested for 2*20 min, vascular parameters SBP (−4.76 mmHg, p < 0.001) and PP (−3.91 mmHg, p < 0.001) were decreased, BAD increased significantly (0.43% mmHg, p < 0.001). Cardiac parameters showed HR was not returned to baseline (p < 0.05), while CO (−0.10 L/min, p < 0.05) and LV dP/dt Max (−91.77 mmHg/s, p < 0.001) were decreased significantly. Blood oxygen and ear temperature returned to baseline (p = 0.051 and p = 0.139, respectively). The corrected p value = the p value * 4 for there are four tests in the multiple comparisons. We claimed significant difference when the corrected p value in Table 2 was smaller than alpha (* alpha = 0.05, † alpha = 0.01, ‡ alpha = 0.001).
Figures 3 and 4 illustrate average and different seasonal trends of blood pressure, vascular, cardiac, blood oxygen and ear temperature parameters. On blood pressure and vascular parameters, decreased blood pressure during bathing for 2*20 min was significant especially in winter (p < 0.001). Pulse pressure increased and brachial artery distensibility decreased (p < 0.01 and p < 0.001, respectively) in summer during bathing for 2*20 min. On cardiac parameters, heart rate, cardiac output and LV dP/dt Max increased especially in summer during bathing for 2*20 min (p < 0.001, p < 0.001, and p < 0.05, respectively). Trends of parameters showed consistent between different seasons.
In Tables 3-1, the univariate and multivariate linear regression models showed the vascular and blood oxygen effects during bathing for 2*20 min in four seasons compared to baseline are as follows: cSBP and cDBP all dropped significantly in winter (cSBP −14.05 mmHg (−10.0%) and cDBP −18.75 mmHg (−22.1%), p < 0.001), cDBP dropped significantly in all four seasons. Blood oxygen showed no significant change. Ear temperature increment was correlated with decreased cSBP (p < 0.01) and cDBP (p < 0.001). Compared to women, men were less likely correlated with decreased cSBP (p < 0.01).
In Tables 3-2, the univariate and multivariate linear regression models showed the cardiac effects during bathing for 2*20 min in four seasons compared to baseline are as follows: HR (20.73 beat/min, +28.4%, p < 0.001), CO (2.64 L/min, +54.9%, p < 0.001), LV dP/dt Max (336.15 mmHg/s, +27.6%, p < 0.01) were all increased significantly in summer. Ear temperature increment was correlated with increased HR (p < 0.001) and CO (p < 0.05). Ambient temperature increment was correlated with increased CO (p < 0.05) and LV dP/dt Max (p < 0.05). In the contrary, hypertension with medication was negatively correlated with HR and CO (p < 0.05), and aging was also negatively correlated with CO and LV dP/dt Max (p < 0.05).
In Tables 4-1, the univariate and multivariate linear regression models showed the vascular and blood oxygen effects after bathing and rested for 2*20 min in all four seasons were resumed to baseline without significant difference.
In Tables 4-2, the univariate and multivariate linear regression models showed the cardiac effects after bathing and rested for 2*20 min in four seasons compared to baseline are as follows: LV dP/dt Max decreased 186.06 mmHg/s in spring (p < 0.05). HR and CO were all increased significantly in summer, autumn, and winter, except for spring without significant change. Ear temperature and ambient temperature increment was correlated with increased HR (p < 0.001 and p < 0.05, respectively). In the contrary, hypertension with medication was still negatively correlated with HR (p < 0.01), and aging was also negatively correlated with HR (p < 0.05).
Environmental and hot spring content assessment (in Supplementary Information) showed that the study spot provided good hot spring quality.
Discussion
This study evaluates cardiovascular effects of hot spring bathing from general population in Northern Taiwan. The hot spring bathing duration and research spot were similar with actual hot spring facility usage habits for Taiwanese population. In Table 2, Our study presents the hidden risk during the bathing process. The most significant effect was decrease in blood pressure during bathing for 2*20 min, both systolic and diastolic blood pressure dropped for around 10 mmHg. Kubota [23] and Ekmekcioglu et al. [3] show short term effect of hot spring bathing on decrease of blood pressure (Kubota et al., −11 mmHg after bathing in 42 °C for 10 min [23]), which reflected the physical effect when human soaked into higher temperature of bathing water than body temperature. Ono et al. reports that during bathing, the decrease in SBP observed 4 min after the start of the bathing (−26 ± 7 mmHg at 39 °C for the older men, and −8 ± 9 mmHg at 39 °C for the younger men) [24]. Dizziness and syncope should always be minded during bathing, and the blood pressure lowering effect may be continued till after hot spring bathing and resting for 2*20 min. Hypertension patients with medication must be suggested to have good medication compliance and follow medical practitioner’s advice before enjoying hot spring bathing. In the aspect of cardiac function, heart rate may be increased up to 20%, and was still above baseline even after 2*20 min bathing and rested for 2*20 min (+1.44 b.p.m., p < 0.05). Kubota et al. demonstrate that the heart rate went back to baseline after 10-min bathing in 42 °C and rested for around 10 min [23]. ŞAŞ et al. presents that the patients with musculoskeletal disease who received 36 °C to 40 °C bathing for 10 days (5 times/week, 20 min/day), the significant increase of heart rate persisted in both 1st and 10th sessions of balneotherapy even after 10 min resting [13]. Though hot-spring bathing for 2*20 min promotes metabolism, the heart burden persisted even after resting or several sessions of bathing should be considered. On vascular function, brachial artery distensibility may be decreased during bathing, which indicates vascular stiffness increased and elasticity decreased. Blood oxygen also showed significant lower during and even maintained until rested for 20 min. Thus, hot spring bathing has potential risks of lowering blood pressure, increased heart rate, increased vascular stiffness, and decreased blood oxygen during bathing. The comprehensive quantitative analysis of physiological changes and potential risks persisted even after bathing (decreased blood pressure, increased heart rate, and decreased blood oxygen) were less stated in previous studies. In the contrary, potential vascular benefit with brachial artery distensibility increased after bathing and 2*20 min resting, which indicated the vascular elasticity increased after hot spring bathing, just as prior study reported by Sato et al. [25]. Decreased CO and cardiac stress (LV dP/dt Max) after bathing and 2*20 min resting were primarily stated in our study.
In our study, we had also tested for physiological changes of cardiovascular system before, during and after hot spring bathing delicately for five times in four seasons (Tables 3-1, 3-2, 4-1, 4-2). Similar trends of functional change in four seasons were presented, however, there were still different effects between four seasons. In the aspect of blood pressure and blood oxygen, there was 14.05 mmHg dropped (−10.0%) in cSBP in winter compared to spring after 2*20 min bathing according to multivariate linear regression analysis (Tables 3-1). This may be related to relatively higher baseline blood pressure for most population in lower environmental temperature than other seasons [26], therefore, the significant decrease of blood pressure in winter should be kept in mind. Kanda et al. also presents significant differences in systolic blood pressure were found between winter and summer before bathing and after bathing conditions [27]. Increased ear temperature may also be a potential risk factor of decreased blood pressure after 2*20 min bathing in our study (Tables 3-1). Ono et al. also demonstrate that in bathing, the rectal temperature gradually increased from baseline with a significant difference. The potential risk in increased central temperature (rectal temperature) and the correlation with decreased blood pressure, especially in elderly during bathing [24], worth further investigations. Blood oxygen showed no significant change after 2*20 min bathing in all seasons (Tables 3-1). After bathing and 2*20 min resting, blood pressure and oxygen in all seasons were no significant difference from baseline (Tables 4-1).
Regarding cardiac function, Kanda et al. presents that no significant differences in heart rate were found between winter and summer at any stage of bathing. Significant differences were found between before and during bathing condition, and between the partially dressed condition and after bathing condition [27]. We monitored CO and LV dP/dt Max for extensive survey for cardiac physiological change (Tables 3-2 and 4-2). In Tables 3-2, according to multivariate linear regression analysis, there were 1.91, 1.54, 1.43 times CO significantly increased after 2*20 min bathing in summer, autumn and winter, compared to spring. In addition, there was 1.69 times LV dP/dt Max significantly increased in summer than in spring. This indicated increased cardiac stress in summer than other seasons, especially after 2*20 min bathing. The cardiac function measurement during hot spring bathing for 20 min (1 cycle) was similar with other seasons, which suggests hot spring bathing in summer should take in shorter duration. Increasing ear and ambient temperature were both risks for increasing cardiac output (CO) after 2*20 min bathing. This may be related to excessive burden on body temperature regulation while soaking in high-temperature hot spring for longer duration in higher surrounding temperature. Ono et al. also show that the older men showed smaller increases in skin after 42 °C bathing and 7 min resting period than the younger men. In addition to the increase in central temperatures faster during bathing in the elderly [24], correlations of ambient temperature and increasing CO may be affected by the thinner skin in the elderly. In Tables 4-2, after bathing and 2*20 min resting in our study, by the interception, LV dP/dt Max significantly decreased 186.06 mmHg/s in spring and there was no significant difference in other seasons compared to spring, which may indicate potential cardiac stress relieving benefit in all seasons. This could be beneficial mechanism with hormesis effect (an adaptive response of the organism to heat [28, 29]) helping with heart failure patients. For example, improvement in the New York Heart Association (NYHA) class, 4.4% of left ventricular ejection fraction, reduction in both SBP and DBP by 3.1% and 5.31%, decrease in cardiothoracic ratio an average of 5.55% reduction, improvement in oxidative stress markers (e.g., BNP, mean difference of 14.8 pg/dL) [30, 31]. Tei et al. studied 34 patients with chronic CHF in NYHA functional class II-IV by echo-Doppler and intracardiac pressures (recorded with right jugular vein Swan-Ganz catheter), hemodynamics improved after warm water or sauna bathing in patients with chronic CHF. This attributed to the reduction in cardiac preload and afterload [32]. Thus, relief of cardiac stress after bathing demonstrated in our study could be reasonably accomplished by this thermal vasodilation effect.
In the aspect of vascular function, pulse pressure increased and brachial artery distensibility decreased significantly in summer after 2*20 min bathing, which indicates increased vascular stiffness during bathing for longer duration in summer (Fig. 3). This also showed vascular health risk if hot spring bathing for longer duration especially in summer. Brachial artery distensibility significantly increased above the average trend in autumn and winter bathing and 2*20 min resting, which indicated increased vascular elasticity after hot spring bathing especially in autumn and winter. (Fig. 3) In conclusion, this study presented CV effects of hot-spring bathing, study significance would be summarized as follows. Comparing the physiological changes in different seasons, the drop of blood pressure was prominent in winter, while increased cardiac workload and vascular stiffness was significant during summer after 2*20 min bathing. Hot-spring bathing could potentially be beneficial to lower cardiac wall stress (presented as LV dP/dt Max in this study) and to increase vascular elasticity especially after bathing and 2*20 min resting. This potentially provides a mechanistic basis for the use of hot spring bathing in patients with CHF. Hot-spring bathing tends to be a physical training process. In addition to seasonal change, body temperature regulation, ambient temperature, hypertension with medication, age and genders may be essential factors for cardiovascular risk management during and after hot spring bathing.
Perspectives
Perspectives of this study indicate that people must be aware of cardiovascular risks during summer and winter, particularly when exposed to extremely cold or hot weather with marked temperature changes. Populations at risk of cardiovascular or chronic lung diseases, should take hot-spring bathing within 1 cycle (below 20 min), due to increased cardiac stress and vascular stiffness were found during bathing for 2*20 min in this study, especially in summer. In winter, they should warm-up themselves indoors prior to hot-spring bathing. Hot-spring bathing CV effects for longer periods of time, in different ethnicities, in different latitudes and different kinds of hot-spring should be further studied. In addition, the hot-spring compounds absorbed from skin may play some roles in CV effects also worth further investigation. That is, overall CV health benefits and risks from hot-spring and the mechanistic basis would further consolidated.
Asian perspectives
The highest incidence of bathtub accidents is in Japan, among 60 countries [6, 8]. That is, this is a public health concern while hot-spring bathing is part of the culture in Asian countries located on the west Circum-Pacific Belt.
Thermal effects on potential cardiovascular risks and benefits after sauna and hot-spring bathing were similar during and after bathing [3, 13, 23,24,25, 30,31,32]. Sauna bathing with hot-water is more common in non-Asian countries, while hot-spring bathing is more prominent in Asian countries. Therefore, the long-term physiological changes in different bathing habits and bathing water mineral contents between sauna and hot-spring bathing also worth further research.
Limitations
Our study investigated seasonal differences of hot-spring bathing on CV effects in middle-aged and older Taiwanese people. However, some limitations apply. First, the experimental design improved during the study process, thus some data collection losses were seen in early study stage. Second, technical error of DynaPulse and poor participant compliance to study process also triggered data collection losses. Third, due to limitations of study duration and sample size, this study focused on short-term hot-spring bathing CV effects during and after hot spring bathing in 2*20 min and failed to track long-term cumulative effects in longer duration or more periods of time. Fourth, the study protocol was restricted by COVID-19 pandemic since January 2020, thus we had lost the second study in winter. Fifth, most importantly, different groups of participants were recruited for each season, intra-individual difference may be amplified. This was mainly due to participants would fail to cooperate with us for long duration physiological tracking, limited choice of the hot-spring temperature, and the research location was a holiday resort far from downtown due to the research fund was limited. Further studies on the same group of participants analyzed over different seasons should be considered.
References
Nasermoaddeli A, Kagamimori S. Balneotherapy in medicine: a review. Environ Health Prev Med. 2005;10:171–9.
Klemenkov SV, Davydova OB, Klemenkova ZHE, Makushkin AK. The effect of carbon dioxide baths on the physical work capacity and extrasystole of patients with ischemic heart disease and stable stenocardia. Vopr kurortologii, fizioterapii, i lechebnoi fizicheskoi Kult. 1995;4:3–5.
Ekmekcioglu C, Strauss-Blasche G, Feyertag J, Klammer N, Marktl W. The effect of balneotherapy on ambulatory blood pressure. Altern Ther Health Med. 2000;6:46–53.
Klemenkov SV, Davydova OB, Levitskiĭ EF, Chashchin NF, Sharova OLA, Kubushko IV. The effect of sodium chloride baths on the physical work capacity and extrasystole of patients with ischemic heart disease and stable stenocardia. Vopr kurortologii, fizioterapii, i lechebnoi fizicheskoi Kult. 1999;3:19–21.
Mancini S Jr, Piccinetti A, Nappi G, Mancini S, Caniato A, Coccheri S. Clinical, functional and quality of life changes after balneokinesis with sulphurous water in patients with varicose veins. VASA. 2003;32:26–30.
Tochihara Y. A review of Japanese-style bathing: its demerits and merits. J Physiol Anthropol. 2022;41:5.
Suzuki M, Shimbo T, Ikaga T, Hori S. Sudden death phenomenon while bathing in Japan—mortality data. Circ J. 2017;81:1144–9.
Lin C-Y, Wang Y-F, Lu T-H, Kawach I. Unintentional drowning mortality, by age and body of water: an analysis of 60 countries. Inj Prev. 2015;21:43–50.
Mendis S, Puska P, Norrving B. Global Atlas on cardiovascular disease prevention and control. World Health Organization. 2011: 3–18.
Thomas H, Diamondy J, Viecoy A, Chaudhuriy S, Shinnary E, Cromery S, et al. Global atlas of cardiovascular disease 2000-2016: the path to prevention and control. Glob Heart. 2018;13:143–63.
Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128.
Cheng JL, MacDonald MJ. Effect of heat stress on vascular outcomes in humans. J Appl Physiol. 2019;126:771–81.
ŞAŞ S, Çelenay ŞT, Kaya DÖ. The effects of balneotherapy on acute, process-related, and cumulative peripheral cardiac responses and pulmonary functions in patients with musculoskeletal disorders. Turk J Med Sci. 2016;46:1700–6.
Ebisu T, Katsuki T, Morita J, Mori K, Yanagimoto Y. Effects of taking a hot spring bath on relaxation. memoirs of the faculty of education and regional studies. Univ Fukui. 2005;4:1–7.
Nobunaga. Adaptability and contraindication of a hot spring cure. Health Care. 1981;23:179–83.
Brinton TJ, Cotter B, Kailasam MT, Brown DL, Chio SS, O’Connor DT, et al. Development and validation of a noninvasive method to determine arterial pressure and vascular compliance. Am J Cardiol. 1997;80:323–30.
Brinton TJ, Walls ED, Chio SS. Validation of pulse dynamic blood pressure measurement by auscultation. Blood Press Monit. 1998;3:121–4.
Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;89:946–51.
Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: the Bogalusa Heart Study. Am J Hypertens. 2005;18:767–71.
Tsao TM, Tsai MJ, Hwang JS, Su TC. Health effects of seasonal variation in cardiovascular hemodynamics among workers in forest environments. Hypertens Res. 2019;42:223–32.
Chio SS, Tsai JJ, Hsu YM, Lapointe JC, Huynh-Covey T, Kwan OL, et al. Development and validation of a noninvasive method to estimate cardiac output using cuff sphygmomanometry. Clin Cardiol. 2007;30:615–20.
Chen SY, Chan CC, Lin YL, Hwang JS, Su TC. Fine particulate matter results in hemodynamic changes in subjects with blunted nocturnal blood pressure dipping. Environ Res. 2014;131:1–5.
Kubota K, Tamura K, Kurabayashi H, Take H, Shirakura T, Tamura J. Effects of hot spring bathing on blood pressure, heart rate, plasma cortisol and hematocrit at Kusatsu. J Jpn Soc Balneol Clim Phys Med. 1997;60:61–68.
Ono J, Hashiguchi N, Sawatari H, Ohkusa T, Miyazono M, Son SY, et al. Effect of water bath temperature on physiological parameters and subjective sensation in older people. Geriatr Gerontol Int. 2017;17:2164–70.
Sato M, Kanikowska D, Iwase S, Nishimura N, Shimizu Y, Belin de Chantemele E, et al. Effects of immersion in water containing high concentrations of CO2 (CO2-water) at thermoneutral on thermoregulation and heart rate variability in humans. Int J Biometeorol. 2009;53:25–30.
Inoue T, Inoue S, Kubota K. Bactericidal activity of manganese and iodide ions against Staphylococcus aureus: a possible treatment for acute atopic dermatitis. Acta Derm Venereol. 1999;79:360–2.
Kanda K, Tsuchiya J, Seto M, Ohnaka T, Tochihara Y. Thermal conditions in the bathroom in winter and summer, and physiological responses of the elderly during bathing. Nihon Eiseigaku Zasshi. 1995;50:595–603.
Davinelli S, Bassetto F, Vitale M, Scapagnini G. Thermal waters and the hormetic effects of hydrogen sulfide on inflammatory arthritis and wound healing. The science of hormesis in health and longevity. Academic Press: Cambridge, MA, USA, 2019 pp. 121–6.
Scapagnini G, Davinelli S, Fortunati N, Zella D, Vitale M. Thermal hydrotherapy as adaptive stress response: hormetic significance, mechanisms, and therapeutic implications. Hormesis in health and disease. Boca Raton, FL: CRC Press; 2014, p. 151–64.
Oyama J, Kudo Y, Maeda T, Node K, Makino N. Hyperthermia by bathing in a hot spring improves cardiovascular functions and reduces the production of inflammatory cytokines in patients with chronic heart failure. Heart Vessels. 2013;28:173–8.
Ye WN, Thipse M, Mahdi MB, Azad S, Davies R, Ruel M, et al. Can heat therapy help patients with heart failure? Artif Organs. 2020;44:680–92.
Tei C, Horikiri Y, Park JC, Jeong JW, Chang KS, Toyama Y, et al. Acute hemodynamic improvement by thermal vasodilation in congestive heart failure. Circulation. 1995;91:2582–90.
Acknowledgements
We express our deepest gratitude to Dr. Hsin-Hsin Tung (Institute of Environmental Engineering, College of Engineering, National Taiwan University, Taipei, Taiwan) and the staff of the Old Jinshan Governor-General Hot Spring for their assistance in this investigation. We acknowledge scientific editing by Jay J. Liao M.D., associate professor in University of Washington Medical Center, Seattle Cancer Care Alliance.
Funding
This study was financially supported by the National Science and Technology Council of Taiwan (106-2314-B-002-073-MY2), the National Taiwan University Hospital Research Ethics Committee (NTUHREC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, PC., Song, QC., Chen, CY. et al. Cardiovascular physiological effects of balneotherapy: focused on seasonal differences. Hypertens Res 46, 1650–1661 (2023). https://doi.org/10.1038/s41440-023-01248-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-023-01248-4
Keywords
This article is cited by
-
Sympathetic nervous activation and hypertension
Hypertension Research (2023)
-
Effects of hot spring bathing on cardiac and vascular function
Hypertension Research (2023)