Abstract
Hot spring bathing is practiced to help manage various diseases, including hypertension. We investigated the preventive effects on hypertension of hot spring bathing among older adults in a data analysis using responses to a previous questionnaire with the aim to identify a novel approach in the prevention and management of hypertension. Among 10,428 adults aged ≥ 65 years, we assessed the hot spring bathing habits of 4001 individuals with a history of hypertension. We calculated odds ratios (OR) with 95% confidence intervals using a multivariable logistic regression model for history of hypertension. In multivariable logistic regression, age (≥ 85 years: OR, 1.410); history of arrythmia (OR, 1.580), stroke (OR, 1.590), gout (OR, 1.880), diabetes mellitus (OR, 1.470), hyperlipidemia (OR, 1.680), renal disease (OR, 1.520), chronic hepatitis (OR, 0.648); and hot spring bathing at 19:00 or later (OR, 0.850) were independently and significantly associated with hypertension during the lifetime. We found an inverse relationship between habitual nighttime hot spring bathing and a history of hypertension. Prospective randomized controlled trials on nighttime hot spring bathing as a treatment for hypertension are warranted to investigate whether nighttime hot spring bathing can help in preventing hypertension among adults aged ≥ 65 years.
Similar content being viewed by others
Introduction
Hypertension is the leading reason for hospital visits and for the long-term use of prescription medications1,2,3. In Japan, ≥ 60% of men aged ≥ 50 years and of women aged ≥ 60 years had hypertension in 20164. In the United States, although the proportion of adults with blood pressure above the target increased from 39 to 53%, the proportion of adults with a recommendation for antihypertensive medication increased from 34 to 36%, according to pooled data from 2011 to 2014. Most patients classified as above require nonpharmacologic intervention as initial therapy2. Additionally, owing to multiple stressors encountered within their occupation, groups including military personnel, firefighters, and police officers have an elevated risk for the development of cardiometabolic diseases such as atherosclerosis5, heart disease6, and sudden cardiac death7, which are related to hypertension8.
Hot spring bathing has expanded globally from Japan and Asia. Maeda et al. reported preventive effects of the occurrence of hypertension in older women9. However, details regarding the relationship between hot spring bathing and hypertension remain unknown.
To address the evidence gap regarding the management of older patients with hypertension, we retrospectively examined the relationship between habitual hot spring bathing and the history of several chronic conditions, including hypertension, in adults aged ≥ 65 years.
Methods
Participant selection
We retrospectively reviewed data from an anonymous questionnaire survey (Supplementary Fig. S1) conducted in 2011 with 11,146 responses from individuals aged ≥ 65 years who were living in Beppu city, Japan9. In that year, there were 34,465 residents of Beppu city in the age group ≥ 65 years. Questionnaires regarding hot spring bathing habits and disease history were randomly sent to 20,000 residents of Beppu. We analyzed responses from a total of 10,429 survey participants regarding age, sex, disease history, and hot spring bathing habits (Table 1). Of these, we analyzed 10,429 participants who provided valid information regarding age, sex, disease history, and hot spring bathing habits, after we checked all responses in the previously developed and described questionnaire. We also reevaluated all questionnaires, including any descriptions in the free-text response area of the questionnaire. The examined variables were as follows: age; sex; disease history during the lifetime including depression, ischemic heart disease, arrhythmia, hypertension, stroke, gout, asthma, diabetes mellitus, hyperlipidemia, renal disease, chronic hepatitis, collagen disease, and allergy; and hot spring bathing habits, including frequency, duration of immersion, years of habitual bathing, typical time of bathing, and type of hot spring. Informed consent to participate in the study was obtained by providing participants with information on our hospital website. The study was performed in accordance with the institutional guidelines and the principles of the Declaration of Helsinki. The protocol was approved by the institutional review board of Kyushu University Hospital, Japan (No. 24–105).
Statistical methods
We analyzed the frequencies and descriptive statistics of participants’ variables. Intergroup differences in categorical variables are expressed as number and percentage. The chi-square statistical method was used to test the relationships between categorical variables. Univariable and multivariable logistic regression models were used to determine associations between variables and the prevalence of hypertension. Covariates that were significant at p < 0.05 in univariate analysis were included in the multivariate analysis. All tests were two-sided. We calculated 95% confidence intervals (CIs), and p < 0.05 was considered statistically significant. Analyses were conducted using EZR (Saitama Medical Center, Saitama, Japan; http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html)10, which is a graphical user interface for R version 2.13.0 (www.r-project.org), and a modified version of R Commander version 1.6–3 designed to add statistical functions.
Ethical approval and Consent to Participate
This was a retrospective study with no experimental interventions. The study was approved by the Institutional Review Board of Kyushu University Hospital in Japan. The study was performed in accordance with the institutional guidelines and principles of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Results
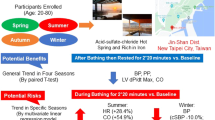
Overall, 4001 of 10,428 participants (38.3%) had a history of hypertension. The baseline characteristics of participants with hypertension during the lifetime are presented in Table 1. In a univariable logistic regression model, age ≥ 85 years (odds ratio [OR], 1.460; 95% CI, 1.230–1.740; p < 0.001), female sex (OR, 0.923; 95% CI, 0.852–0.999; p = 0.048), arrhythmia (OR, 1.610; 95% CI, 1.400–1.850; p < 0.001), stroke (OR, 1.620; 95% CI, 1.310–2.010; p < 0.001), gout (OR, 1.860; 95% CI, 1.310–2.010; p < 0.001), diabetes mellitus (OR, 1.460; 95% CI, 1.300–1.630; p < 0.001), hyperlipidemia (OR, 1.650; 95% CI, 1.460–1.870; p < 0.001), renal disease (OR, 1.520; 95% CI, 1.230–1.880; p < 0.001), chronic hepatitis (OR, 0.656; 95% CI, 0.500–0.859; p = 0.002), hot spring bathing frequency (2–3 times/week: OR, 1.200; 95% CI, 1.040–1.390; p = 0.013 and daily: OR, 0.868; 95% CI, 0.790–0.954; p = 0.003), duration of immersion (10–19 min: OR, 0.832; 95% CI, 0.753–0.918; p < 0.001 and 20–29 min: OR, 0.765; 95% CI, 0.652–0.897; p < 0.001)), bathing time (13:00 to 19:00: OR, 1.220; 95% CI, 1.110–1.330; p < 0.001 and 19:00 or later: OR, 0.840; 95% CI, 0.738–0.957; p = 0.008), and years of habitual hot spring bathing (10–19 years: OR, 0.851; 95% CI, 0.745–0.973; p = 0.017) were significantly associated with influencing hypertension during the lifetime (Table 2). In a multivariable logistic regression model, age (≥ 85 years: OR, 1.410; 95% CI, 1.170–1.680; p < 0.001), history of arrythmia (OR, 1.580; 95% CI, 1.380–1.810; p < 0.001), stroke (OR, 1.590; 95% CI, 1.280–1.980; p < 0.001), gout (OR, 1.880; 95% CI, 1.530–2.310; p < 0.001), diabetes mellitus (OR, 1.470; 95% CI, 1.310–1.650; p < 0.001), hyperlipidemia (OR, 1.680; 95% CI, 1.480–1.910; p < 0.001), renal disease (OR, 1.520; 95% CI, 1.230–1.880; p < 0.001), chronic hepatitis (OR, 0.648; 95% CI, 0.494–0.851; p = 0.001); and hot spring bathing at 19:00 or later (OR, 0.850; 95% CI, 0.768–0.940; p = 0.001) were independently and significantly associated with the prevalence of hypertension during the lifetime (Table 2). These findings support the hypothesis that hypertension can be influenced by habitual nighttime hot spring bathing.
Discussion
Traditional thermal therapy and hot spring bathing have proven useful for various diseases, including hypertension11. We investigated the preventive effects of long-term hot spring bathing in adults aged ≥ 65 years. We found that age ≥ 85 years; history of arrythmia, stroke, gout, diabetes mellitus, hyperlipidemia, and renal disease were independently and significantly associated with a higher risk of developing hypertension during the lifetime. We found that a history of chronic hepatitis and hot spring bathing time were independently and significantly protective against hypertension development during the lifetime. These results support our hypothesis that habitual nighttime hot spring bathing is protective against hypertension development.
The implications of our data can be extrapolated regarding the prevalence of hypertension according to questionnaire survey responses from adults aged ≥ 65 years. We found that nighttime hot spring bathing, which can improve sleep disorders, might be inversely associated with a history of hypertension in adults aged ≥ 65 years. In a large-scale study among an older population, Tai et al. reported that nighttime hot spring bathing was significantly associated with shorter sleep onset latency, if bathing is scheduled 1–3 h before bedtime, and higher distal–proximal skin temperature gradient if bathing takes place 30 min before bedtime12. Sawatari et al. suggested that leg thermal therapy could improve subjective and objective sleep quality in patients with chronic heart failure13. The COVID-19 pandemic has placed all people at risk for developing psychiatric and mental health disorders, including sleep disturbances14. It is therefore possible that nighttime hot spring bathing may improve sleep, which may result in improved hypertension control15.
According to our data, age ≥ 85 years was significantly associated with the prevalence of hypertension. Many risk factors are associated with hypertension development, such as age, obesity, family history, high-sodium diet, and physical inactivity16,17. Disease history, stroke18, and renal disease19,20 are associated with hypertension. Given that health care spending on hypertension exceeded USD 70 billion in the United States between 1996 and 201621, clinicians and researchers have great interest in proactive and preventive interventions versus reactive approaches for hypertension. In this study, we demonstrated that an alternative option for potentially improving hypertension control in adults aged ≥ 65 years is habitual nighttime hot spring bathing.
Stress has two components: an acute phase and a chronic phase22. Rozanski et al. verified a direct association between cardiovascular disease and chronic stress, which is known to modulate vascular endothelial cell function and platelet aggregation23. Dual stressors include psychological and physiological stressors and are known to elicit activation of the hypothalamic and sympathoadrenal axes, with a subsequent greater release of stress markers such as cortisol, epinephrine, and norepinephrine, in comparison with a single stressor24,25. Increased levels of cortisol and oxidative stress in the body can upregulate several proinflammatory pathways, which can result in the development of several cardiovascular diseases including hypertension26. Endocrine responses to sauna bathing show that some markers of stress, such as cortisol, β-endorphins, and adrenocorticotropic hormone, respond to acute heat exposure in a highly variable manner27. Different results regarding the hormone response are likely owing to differences in study methods and consideration of factors such as therapy duration, time, and frequency, which were considered in our study. Therefore, understanding the cardiovascular responses will provide a more comprehensive picture of the physiological responses to hot spring bathing. Blood pressure after a sauna bath appears decreased compared with that before a sauna bath28. Although brief exposure to sauna baths can result in benefits for < 1 h, including reduced blood pressure and improved arterial stiffness, sauna bath exposure for ≥ 3 weeks and repeated frequency can upregulate enzymes and pathways, which results in greater stress tolerance, an enhanced cellular environment, and improved health29. Owing to a lack of physical activity and healthy nutrition and with time pressure among individuals aged ≥ 65 years, practical interventions that can prevent or improve hypertension, such as habitual hot spring bathing, warrant additional attention.
Our study has some limitations that should be acknowledged. First, some selection bias is expected with use of questionnaire surveys. In this study, bias is present owing to differences in data selection and other factors, including a lack of data regarding hypertensive patients who engaged in hot spring bathing for the treatment of hypertension; participant incomes, which might be correlated with some vascular diseases or the frequency of hot spring bathing; and regarding participants’ lifestyle, such as diet, physical activity, and sleep. Second, important data regarding the prevalence of hypertension are likely missing because the information was collected using self-report questionnaires. To minimize bias, we limited the inclusion criteria to age, sex, disease history, and hot spring bathing habits. Third, we have no data about the treatment and outcomes of hypertension; further studies are needed to assess the details of treatment and outcomes in patients with hypertension. Fourth, a history of hypertension may have been overlooked or underreported in some study participants because of the self-report nature of the questionnaire; moreover, diagnoses of disease history, including hypertension, were not confirmed by a physician. Finally, the purpose of this study was to help clarify the relationship between the prevention of hypertension and habitual hot spring bathing. However, it was difficult to interpret the obtained evidence, such as the interactions among duration of immersion, frequency, time, and years of habitual hot spring bathing, because we did not evaluate the quality of the questionnaire data.
Conclusions
In this study, we found that habitual nighttime hot spring bathing was significantly associated with a lower prevalence of hypertension in older adults. It is important to prioritize clinical trials regarding the prevalence of hypertension, including identifying effective approaches to the monitoring and management of arrhythmia, stroke, gout, diabetes mellitus, hyperlipidemia, renal disease, and chronic hepatitis. Randomized controlled trials on habitual nighttime hot spring bathing as a treatment for hypertension are warranted.
Data availability
We used data obtained from a questionnaire performed in 2011 in Beppu, Japan. The datasets generated and analyzed during the current study are not publicly available owing to privacy and confidentiality restrictions pertaining to personal health information. However, the dataset creation plan is available from the corresponding author on reasonable request.
Change history
22 December 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41598-022-26365-x
References
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the American college of cardiology/American heart association task force on clinical practice guidelines. Hypertension 71, e13–e115 (2018).
Muntner, P. et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 137, 109–118 (2018).
Yoon, S. S. et al. Trends in blood pressure among adults with hypertension: United States, 2003 to 2012. Hypertension 65, 54–61 (2015).
Hisamatsu, T. et al. Epidemiology of hypertension in Japan: Beyond the new 2019 Japanese guidelines. Hypertens. Res. 43, 1344–1351 (2020).
Joseph, P. N. et al. Police work and subclinical atherosclerosis. J. Occup. Environ. Med. 51, 700–707 (2009).
Kales, S. N., Soteriades, E. S., Christoudias, S. G. & Christiani, D. C. Firefighters and on-duty deaths from coronary heart disease: A case control study. Environ Health 2, 14 (2003).
Yang, J. et al. Sudden cardiac death among firefighters 45 years of age in the United States. Am. J. Cardiol. 112, 1962–1967 (2013).
Hurtubise, J. et al. The different facets of dyslipidemia and hypertension in atherosclerosis. Curr. Atheroscler. Rep. 18, 82 (2016).
Maeda, T., Mimori, K., Suzuki, S., Horiuchi, T. & Makino, N. Preventive and promotive effects of habitual hot spa-bathing on the elderly in Japan. Sci. Rep. 8, 133 (2018).
Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 48, 452–458 (2013).
Henderson, K. N., Killen, L. G., O’Neal, E. K. & Waldman, H. S. The cardiometabolic health benefits of sauna exposure in individuals with high-stress occupations a mechanistic review. Int. J. Environ. Res. Public Health 18, 1105 (2021).
Tai, Y. et al. Hot-water bathing before bedtime and shorter sleep onset latency are accompanied by a higher distal-proximal skin temperature gradient in older adults. J. Clin. Sleep Med. 17, 1257–1266 (2021).
Sawatari, H. et al. Three nights leg thermal therapy could improve sleep quality in patients with chronic heart failure. Heart Vessels 33, 155–162 (2018).
Deng, J. et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: A meta-analysis. Ann. N Y Acad. Sci. 1486, 90–111 (2021).
Haghayegh, S., Khoshnevis, S., Smolensky, M. H., Diller, K. R. & Castriotta, R. J. Before-bedtime passive body heating by warm shower or bath to improve sleep: A systematic review and meta-analysis. Sleep Med Rev 46, 124–135 (2019).
Forman, J. P., Stampfer, M. J. & Curhan, G. C. Diet and lifestyle risk factors associated with incident hypertension in women. JAMA 302, 401–411 (2009).
Carnethon, M. R. et al. Joint associations of physical activity and aerobic fitness on the development of incident hypertension: Coronary artery risk development in young adults. Hypertension 56, 49–55 (2010).
Staessen, J. A. et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension the systolic hypertension in Europe (Syst-Eur) trial investigators. Lancet 350, 757–764 (1997).
Coresh, J. et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: Findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch. Intern. Med. 161, 1207–1216 (2001).
Bansal, N. et al. Blood pressure and risk of cardiovascular events in patients on chronic hemodialysis: The CRIC study (Chronic Renal Insufficiency Cohort). Hypertension 70, 435–443 (2017).
Birger, M. et al. Spending on cardiovascular disease and cardiovascular risk factors in the United States: 1996 to 2016. Circulation 144, 271–282 (2021).
Rohleder, N. Stress and inflammation—The need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology 105, 164–171 (2019).
Rozanski, A., Blumenthal, J. A. & Kaplan, J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation 99, 2192–2217 (1999).
Huang, C. J. et al. Psychological stress during exercise: Immunoendocrine and oxidative responses. Exp. Biol. Med. (Maywood) 235, 1498–1504 (2010).
Webb, H. E. et al. Psychological stress during exercise: Cardiorespiratory and hormonal responses. Eur. J. Appl. Physiol. 104, 973–981 (2008).
Pinheiro, L. C. & Oliveira-Paula, G. H. Sources and effects of oxidative stress in hypertension. Curr. Hypertens. Rev. 16, 166–180 (2020).
Podstawski, R., Borysławski, K., Pomianowski, A., Krystkiewicz, W. & Żurek, P. Endocrine effects of repeated hot thermal stress and cold water immersion in young adult men. Am. J. Mens. Health 15, 15579883211008340 (2021).
Ketelhut, S. & Ketelhut, R. G. The blood pressure and heart rate during sauna bath correspond to cardiac responses during submaximal dynamic exercise. Complement Ther. Med. 44, 218–222 (2019).
Henderson, K. N., Killen, L. G., O’Neal, E. K. & Waldman, H. S. The cardiometabolic health benefits of Sauna exposure in individuals with high-stress occupations a mechanistic review. Int. J. Environ. Res. Public Health 18, 1105 (2021).
Acknowledgements
We thank the patients and clinical staff for their participation in the study. We thank Analisa Avila, MPH, ELS, of Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors.
Author information
Authors and Affiliations
Contributions
S. Y. designed the study, analyzed the data, and prepared the manuscript. S. Y., T. T., T. M., and T. H. prepared and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this Article was revised: The original version of this Article contained an error in the Abstract. “We found an inverse relationship between habitual nighttime hot spring bathing and a history of depression.” now reads: “We found an inverse relationship between habitual nighttime hot spring bathing and a history of hypertension.”
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamasaki, S., Tokunou, T., Maeda, T. et al. Hot spring bathing is associated with a lower prevalence of hypertension among Japanese older adults: a cross-sectional study in Beppu. Sci Rep 12, 19462 (2022). https://doi.org/10.1038/s41598-022-24062-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-24062-3
This article is cited by
-
Effects of bathing in different hot spring types on Japanese gut microbiota
Scientific Reports (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.