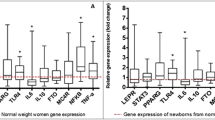
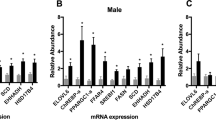
Abstract
Background
Maternal body size, nutrition, and hyperglycemia contribute to neonatal body size and composition. There is little information on maternal-fetal transmission of messages which influence fetal growth. We analyzed adipocyte-derived small extracellular vesicular (ADsEV) microRNAs in maternal and cord blood to explore their adipogenic potential.
Methods
There were 279 mother-neonate pairs with all phenotypic data (normal glucose tolerant NGT = 148, gestational diabetes mellitus GDM = 131). Neonates with adiposity were those in the highest tertile (T3) of sex-specific sum of skinfolds and those without adiposity (lean) in the lowest tertile T1 of NGT pregnancies. We studied ADsEV miRNAs in 76 and 51 neonates with and without adiposity respectively and their mothers based on power calculations (68 NGT and 59 GDM pregnancies). ADsEV miRNAs from maternal and cord blood plasma samples were profiled on Agilent 8*60 K microarray. Differential expression (DE) of ADsEV miRNAs in adipose vs. lean groups was studied before and after adjustment for maternal GDM, adiposity, and vitamin B12-folate status.
Results
Multiple miRNAs were common in maternal and cord blood and positively correlated. We identified 24 maternal and 5 cord blood miRNAs differentially expressed (discovery p ≤ 0.1) in the adipose group in unadjusted, and 19 and 26, respectively, in the adjusted analyses. Even though DE miRNAs were different in maternal and cord blood, they targeted similar adipogenic pathways (e.g., the forkhead box O (FOXO) family of transcription factors, mitogen‑activated protein kinase (MAPK) pathway, transforming growth factor beta (TGF-β) pathway). Maternal GDM and adiposity were associated with many DE ADsEV miRNAs.
Conclusion
Our results suggest that the ADsEV miRNAs in mothers are potential regulators of fetal adiposity. The expression and functionality of miRNAs appear to be influenced by maternal adiposity, hyperglycemia, and micronutrient status during pregnancy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The raw microarray data is available at Gene Expression Omnibus (Accession ID: GSE217933). The demographic, and phenotypic data is available with the authors and can be made available for a reasonable request following institutional clearances.
Code availability
Code used for calculating differential expression of miRNAs can be accessed from GitHub on the following address: https://github.com/poojakunte24/IJO_ADsEV_manuscript/tree/f16066c106f8ed7f7725b8d4eba67cc14646b2b8.
References
Williams RH, Larsen PR. Chapter 20. Endocrine Changes in Pregnancy. 10 ed. Williams textbook of endocrinology: Philadelphia, Pa. : Saunders; (2003).
Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. 1992. Int J Epidemiol. 2013;42:1215–22.
Yajnik CS, Deshmukh US. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev Endocr Metab Disord. 2008;9:203–11.
Prachi Katre CSY. Pune Experience. Influence of early life environment on risk of non-nommunicable diseases (NCDs) in Indians. SIGHT AND LIFE. 2015;19.
Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, et al. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80.
Yajnik CS, Lubree HG, Rege SS, Naik SS, Deshpande JA, Deshpande SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87:5575–80.
Yajnik CS, Yajnik PC. Fetal adiposity epidemic in the modern world: a thrifty phenotype aggravated by maternal obesity and diabetes. Am J Clin Nutr. 2020;112:8–10.
D’Angelo S, Yajnik CS, Kumaran K, Joglekar C, Lubree H, Crozier SR, et al. Body size and body composition: a comparison of children in India and the UK through infancy and early childhood. J Epidemiol Community Health. 2015;69:1147–53.
Bavdekar A, Yajnik CS, Fall CH, Bapat S, Pandit AN, Deshpande V, et al. Insulin resistance syndrome in 8-year-old Indian children: small at birth, big at 8 years, or both? Diabetes. 1999;48:2422–9.
Yajnik CS, Deshpande SS, Jackson AA, Refsum H, Rao S, Fisher DJ, et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia. 2008;51:29–38.
Kaspar D, Hastreiter S, Irmler M, Hrabe de Angelis M, Beckers J. Nutrition and its role in epigenetic inheritance of obesity and diabetes across generations. Mamm Genome. 2020;31:119–33.
Reichetzeder C. Overweight and obesity in pregnancy: their impact on epigenetics. Eur J Clin Nutr. 2021;75:1710–22.
Wang K, Li WD, Zhang CK, Wang Z, Glessner JT, Grant SF, et al. A genome-wide association study on obesity and obesity-related traits. PLoS One. 2011;6:e18939.
Czernek L, Duchler M. Exosomes as Messengers Between Mother and Fetus in Pregnancy. Int J Mol Sci. 2020;21.
Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE, et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204.
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750.
Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature. 2017;542:450–5.
Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–92.
Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77:447–54.
Hubal MJ, Nadler EP, Ferrante SC, Barberio MD, Suh JH, Wang J, et al. Circulating adipocyte-derived exosomal MicroRNAs associated with decreased insulin resistance after gastric bypass. Obesity (Silver Spring). 2017;25:102–10.
Hromadnikova I, Kotlabova K, Dvorakova L, Krofta L, Sirc J. Substantially Altered Expression Profile of Diabetes/Cardiovascular/Cerebrovascular Disease Associated microRNAs in Children Descending from Pregnancy Complicated by Gestational Diabetes Mellitus-One of Several Possible Reasons for an Increased Cardiovascular Risk. Cells. 2020;9.
Xie Z, Wang X, Liu X, Du H, Sun C, Shao X, et al. Adipose-Derived Exosomes Exert Proatherogenic Effects by Regulating Macrophage Foam Cell Formation and Polarization. J Am Heart Assoc. 2018;7.
Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–808.
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–30.
Sonali S Wagle SP, Bhat D, Kalyanaraman K, Rajashree K, Sayali W, et al. Adiposity and Glucose Intolerance in Offspring of Diabetic Mothers in India. medRxiv. 2021.
International Association of D, Pregnancy Study Groups Consensus P, Metzger BE, Gabbe SG, Persson B, Buchanan TA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33:676–82.
Warnes GR LP, Li F. ssize: Estimate Microarray Sample Size. 2023.
Wickham H. ggplot2: Elegant Graphics for Data Analysis 2016 [Available from: https://ggplot2.tidyverse.org.
Licursi V, Conte F, Fiscon G, Paci P. MIENTURNET: an interactive web tool for microRNA-target enrichment and network-based analysis. BMC Bioinformatics. 2019;20:545.
Moritz Marbach FB, Heike H, Pedro J, Ming-Yu L. ggnet2 - Plot a network with ggplot2.
Statnet Development Team. Krivitsky PN, Handcock MS, Hunter DR, Butts CT, Klumb C, Goodreau SM, Morris M (2003-2023). statnet: Software tools for the Statistical Modeling of Network Data. http://statnet.org.
Bogart S. SankeyMATIC: A Sankey diagram builder for everyone [Available from: https://sankeymatic.com/.
Bardou P, Mariette J, Escudie F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293.
Ferland-McCollough D, Fernandez-Twinn DS, Cannell IG, David H, Warner M, Vaag AA, et al. Programming of adipose tissue miR-483-3p and GDF-3 expression by maternal diet in type 2 diabetes. Cell Death Differ. 2012;19:1003–12.
Nardelli C, Iaffaldano L, Pilone V, Labruna G, Ferrigno M, Carlomagno N, et al. Changes in the MicroRNA Profile Observed in the Subcutaneous Adipose Tissue of Obese Patients after Laparoscopic Adjustable Gastric Banding. J Obes. 2017;2017:6754734.
Joglekar MV, Kunte PS, Wong WKM, Bhat DS, Satoor SN, Patil RR, et al. Circulating microRNAs from early childhood and adolescence are associated with pre-diabetes at 18 years of age in women from the PMNS cohort. J Dev Orig Health Dis. 2022;13:806–11.
Wang L, Xu L, Xu M, Liu G, Xing J, Sun C, et al. Obesity-Associated MiR-342-3p Promotes Adipogenesis of Mesenchymal Stem Cells by Suppressing CtBP2 and Releasing C/EBPalpha from CtBP2 Binding. Cell Physiol Biochem. 2015;35:2285–98.
He H, Chen K, Wang F, Zhao L, Wan X, Wang L, et al. miR-204-5p promotes the adipogenic differentiation of human adipose-derived mesenchymal stem cells by modulating DVL3 expression and suppressing Wnt/beta-catenin signaling. Int J Mol Med. 2015;35:1587–95.
Li G, Fu S, Chen Y, Jin W, Zhai B, Li Y, et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int J Mol Sci. 20 (2019).
Suchacki KJ, Stimson RH. Nutritional Regulation of Human Brown Adipose Tissue. Nutrients. 13 (2021).
Bakker LE, Boon MR, van der Linden RA, Arias-Bouda LP, van Klinken JB, Smit F, et al. Brown adipose tissue volume in healthy lean south Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol. 2014;2:210–7.
Chang YJ, Li YS, Wu CC, Wang KC, Huang TC, Chen Z, et al. Extracellular MicroRNA-92a Mediates Endothelial Cell-Macrophage Communication. Arterioscler Thromb Vasc Biol. 2019;39:2492–504.
Machado SA, Pasquarelli-do-Nascimento G, da Silva DS, Farias GR, de Oliveira Santos I, Baptista LB, et al. Browning of the white adipose tissue regulation: new insights into nutritional and metabolic relevance in health and diseases. Nutr Metab (Lond). 2022;19:61.
Zhu Z, Cao F, Li X. Epigenetic Programming and Fetal Metabolic Programming. Front Endocrinol (Lausanne). 2019;10:764.
Sebo ZL, Jeffery E, Holtrup B, Rodeheffer MS A mesodermal fate map for adipose tissue. Development. 145 (2018).
Ambele MA, Dhanraj P, Giles R, Pepper MS Adipogenesis: A Complex Interplay of Multiple Molecular Determinants and Pathways. Int J Mol Sci. 21 (2020).
Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242–58.
Samblas M, Milagro FI, Martinez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14:421–44.
Crocker KC, Domingo-Relloso A, Haack K, Fretts AM, Tang WY, Herreros M, et al. DNA methylation and adiposity phenotypes: an epigenome-wide association study among adults in the Strong Heart Study. Int J Obes (Lond). 2020;44:2313–22.
Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96.
Yajnik CS. Transmission of obesity-adiposity and related disorders from the mother to the baby. Ann Nutr Metab. 2014;64:8–17. Suppl 1
Fowden AL, Hill DJ. Intra-uterine programming of the endocrine pancreas. Br Med Bull. 2001;60:123–42.
Pedersen J. Diabetes and pregnancy; blood sugar of newborn infants during fasting and glucose administration. Ugeskr Laeger. 1952;114:685.
Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–35.
Acknowledgements
The authors thank Deepa Raut, Neelam Memane, Sayali Wadke and Rajashree Kamat for their help in processing cord blood samples and laboratory assays. Madhura Deshmukh for help in study coordination. Mrs. Pallavi Yajnik and Rasika Ladkat for administrative help. Dr. Leelavati Narlikar for her guidance in data analysis. Dr. Satyajit Rath for his advice in writing the discussion. We also thank the co-investigators of the InDiaGDM study Dr. Giriraj Chandak, Kalyanaraman Kumaran, Dr. Gundu Rao for their valuable contribution in conducting the InDiaGDM study.
Funding
The work was supported by InDiaGDM grant of the Department of Biotechnology, New Delhi, India (BT/IN/Denmark/02/CSY/2014) and National Institute of Health (NIH), the U.S government (R21HD094127-01).
Author information
Authors and Affiliations
Contributions
CSY designed the InDiaGDM study. CSY and HD contributed to managing the clinical cohort. DB, RKK, BH, ES, MB contributed to exosome extraction and protocol standardization. SR contributed to microRNA data generation. PSK, PT, MB, RKK, KA, MG contributed to the data analysis. PSK, MB, CSY, and RF contributed to the manuscript writing. CSY and RF contributed to the discussion and interpretation of the results. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kunte, P., Barberio, M., Tiwari, P. et al. Neonatal adiposity is associated with microRNAs in adipocyte-derived extracellular vesicles in maternal and cord blood, a discovery analysis. Int J Obes 48, 403–413 (2024). https://doi.org/10.1038/s41366-023-01432-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-023-01432-z