Abstract
Background
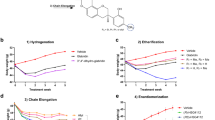
Retinoic acid (RA) controls diverse physiological functions including weight regulation and energy metabolism. It has been reported that mice lacking ALDH1A1, one of the aldehyde dehydrogenases (ALDH) that synthesize RA, are healthy and resistant to weight gain, raising the possibility that inhibiting this enzyme might treat obesity. We previously demonstrated that treatment with a pan-ALDH1A enzyme inhibitor, WIN18446, suppressed weight gain in mice fed a high-fat diet (HFD), but caused increased hepatic lipidosis and reversible male infertility.
Methods
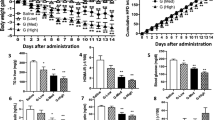
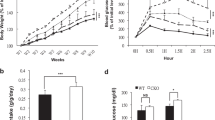
A series of piperazine compounds that inhibited ALDH1A1 were identified and their inhibitory activity was characterized in vitro using purified recombinant enzymes and cell-based assay systems. One potent compound, FSI-TN42 (N42) was examined for its oral bioavailability and pharmacodynamic effects. In addition, its effect on weight gain was investigated by daily oral administration to C57BL/6 male mice receiving a HFD, and compared with mice receiving WIN18446 or vehicle alone (n = 6/group, 200 mg compound/kg body weight) for 5 weeks. Body weights were measured weekly, and a glucose tolerance test was performed after 4 weeks of treatment. Tissues were collected to determine changes in adipose weight, hepatic lipidosis, retinoid metabolism, and expression of genes associated with RA and lipid metabolism.
Results
N42 irreversibly binds and inhibits ALDH1A1 in vitro with a low nM IC50 and 800-fold specificity for ALDH1A1 compared to ALDH1A2. Daily oral administration of N42 significantly suppressed weight gain (P < 0.05) and reduced visceral adiposity (p < 0.05) in mice fed a HFD without the hepatic lipidosis observed with WIN18446 treatment.
Conclusions
We developed a potent and specific inhibitor of ALDH1A1 that suppressed weight gain in mice fed a HFD. These findings demonstrate that inhibition of ALDH1A1 is a feasible target for drug development to treat and/or prevent obesity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99:565–79.
Apovian CM. Obesity: definition, comorbidities, causes, and burden. Am J Manag Care. 2016;22 7 Suppl:s176–85.
Jackson VM, Breen DM, Fortin JP, Liou A, Kuzmiski JB, Loomis AK, et al. Latest approaches for the treatment of obesity. Expert Opin Drug Discov. 2015;10:825–39.
Kushner RF. Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog Cardiovasc Dis. 2018;61:246–52.
Velazquez A, Apovian CM. Updates on obesity pharmacotherapy. Ann N Y Acad Sci. 2018;1411:106–19.
Blaner WS. Vitamin A signaling and homeostasis in obesity, diabetes, and metabolic disorders. Pharmacol Ther. 2019;197:153–78.
Zhang R, Wang Y, Li R, Chen G. Transcriptional factors mediating retinoic acid signals in the control of energy metabolism. Int J Mol Sci. 2015;16:14210–44.
Cañete A, Cano E, Muñoz-Chápuli R, Carmona R. Role of Vitamin A/Retinoic Acid in Regulation of Embryonic and Adult Hematopoiesis. Nutrients. 2017;9:159.
Pino-Lagos K, Benson MJ, Noelle RJ. Retinoic acid in the immune system. Ann N Y Acad Sci. 2008;1143:170–87.
Ghyselinck NB, Duester G. Retinoic acid signaling pathways. Development. 2019;146:dev167502.
Yadu N, Kumar PG. Retinoic acid signaling in regulation of meiosis during embryonic development in mice. Genesis. 2019;57:e23327.
Teletin M, Vernet N, Ghyselinck NB, Mark M. Roles of retinoic acid in germ cell differentiation. Curr Top Dev Biol. 2017;125:191–225.
Kiefer FW, Vernochet C, O’Brien P, Spoerl S, Brown JD, Nallamshetty S, et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat Med. 2012;18:918–25.
Yang D, Krois CR, Huang P, Wang J, Min J, Yoo HS, et al. Raldh1 promotes adiposity during adolescence independently of retinal signaling. PLoS ONE. 2017;12:e0187669.
Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702.
Haenisch M, Treuting PM, Brabb T, Goldstein AS, Berkseth K, Amory JK, et al. Pharmacological inhibition of ALDH1A enzymes suppresses weight gain in a mouse model of diet-induced obesity. Obes Res Clin Pract. 2018;12:93–101.
Paik J, Haenisch M, Muller CH, Goldstein AS, Arnold S, Isoherranen N, et al. Inhibition of retinoic acid biosynthesis by the bisdichloroacetyldiamine WIN 18,446 markedly suppresses spermatogenesis and alters retinoid metabolism in mice. J Biol Chem. 2014;289:15104–17.
Arnold SL, Kent T, Hogarth CA, Schlatt S, Prasad B, Haenisch M, et al. Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations. J Lipid Res. 2015;56:342–57.
Paik J, Fierce Y, Drivdahl R, Treuting PM, Seamons A, Brabb T, et al. Effects of murine norovirus infection on a mouse model of diet-induced obesity and insulin resistance. Comp Med. 2010;60:189–95.
Amory JK, Muller CH, Shimshoni JA, Isoherranen N, Paik J, Moreb JS, et al. Suppression of spermatogenesis by bisdichloroacetyldiamines is mediated by inhibition of testicular retinoic acid biosynthesis. J Androl. 2011;32:111–9.
Chen Y, Zhu JY, Hong KH, Mikles DC, Georg GI, Goldstein AS, et al. Structural basis of ALDH1A2 inhibition by irreversible and reversible small molecule inhibitors. ACS Chem Biol. 2018;13:582–90.
Lamb AL, Newcomer ME. The structure of retinal dehydrogenase type II at 2.7 A resolution: implications for retinal specificity. Biochemistry. 1999;38:6003–11.
Arnold SL, Kent T, Hogarth CA, Griswold MD, Amory JK, Isoherranen N. Pharmacological inhibition of ALDH1A in mice decreases all-trans retinoic acid concentrations in a tissue specific manner. Biochem Pharmacol. 2015;95:177–92.
Raverdeau M, Gely-Pernot A, Feret B, Dennefeld C, Benoit G, Davidson I, et al. Retinoic acid induces Sertoli cell paracrine signals for spermatogonia differentiation but cell autonomously drives spermatocyte meiosis. Proc Natl Acad Sci USA. 2012;109:16582–7.
Kane MA, Chen N, Sparks S, Napoli JL. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem J. 2005;388:363–9.
Kane MA, Folias AE, Wang C, Napoli JL. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal Chem. 2008;80:1702–8.
Acknowledgements
This work was supported by the National Institute of Health (NIH) grant (R56 DK110239-01A1). Protein mass analysis was performed at Mass-Spectrometry Center (School of Pharmacy, UW) and liver histology was performed at Histology Imaging Core (Department of Comparative Medicine, UW).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors (MH, ASG, JKA, PT, and JP) have filed a patent application (WO2020123855) for FSI-TN42. TN, CAF, and TB declare no potential competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Haenisch, M., Nguyen, T., Fihn, C.A. et al. Investigation of an ALDH1A1-specific inhibitor for suppression of weight gain in a diet-induced mouse model of obesity. Int J Obes 45, 1542–1552 (2021). https://doi.org/10.1038/s41366-021-00818-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-021-00818-1
This article is cited by
-
Quantitative proteomics analysis based on data-independent acquisition reveals the effect of Shenling Baizhu powder (SLP) on protein expression in MAFLD rat liver tissue
Clinical Proteomics (2023)
-
Altered macronutrient composition and genetics influence the complex transcriptional network associated with adiposity in the Collaborative Cross
Genes & Nutrition (2022)