Abstract
Objectives
Obesity, an emerging global health issue, involves numerous factors; understanding its underlying mechanisms for prevention and therapeutics is urgently needed. Cellular retinoic acid binding protein 1 (Crabp1) knockout (CKO) mice exhibit an obese phenotype under normal diet (ND) feedings, which prompted us to propose that Crabp1 could play a role in modulating adipose tissue development/homeostasis. Studies were designed to elucidate the underlying mechanism of Crabp1′s action in reducing obesity.
Subjects/methods
In animal studies, 6 weeks old male wild type and CKO mice were fed with ND or high-fat diet (HFD) for 10 weeks. Body weight and food intake were regularly monitored. Glucose tolerance test and biological parameters of plasma (glucose and insulin levels) were measured after 10 weeks of ND vs. HFD feedings. Visceral adipose tissues were collected for histological and molecular analyses to determine affected signaling pathways. In cell culture studies, the 3T3L1 adipocyte differentiation model was used to examine and validate relevant signaling pathways.
Results
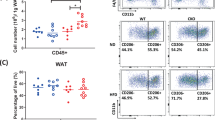
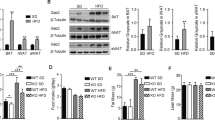
CKO mice, compared to WT mice, gained more body weight, exhibited more elevated fasting plasma glucose levels, and developed more severe impaired glucose tolerance under both ND and HFD. Histological examination revealed readily increased adipocyte hypertrophy and adipose tissue inflammation under HFD feedings. In 3T3L1 adipocytes, Crabp1 silencing enhanced extracellular signal-regulated kinase 1/2 (ERK1/2) activation, accompanied by elevated markers and signaling pathways of lipid accumulation and adipocyte hypertrophy.
Conclusions
This study identifies Crabp1′s physiological role against the development of obesity. The protective function of CRABP1 is likely attributed to its classically proposed (canonical) activity as a trap for RA, which will reduce RA availability, thereby dampening RA-stimulated ERK1/2 activation and adipocyte hypertrophy. The results suggest Crabp1 as a potentially new therapeutic target in managing obesity and metabolic diseases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–54.
Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–81.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107.
Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–77.
Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLoS Comput Biol. 2009;5:e1000324.
Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141–50.
Sarjeant K, Stephens JM. Adipogenesis. Cold Spring Harb Perspect Biol. 2012;4:a008417.
Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–96.
Chen N, Wang J. Wnt/beta-catenin signaling and obesity. Front Physiol. 2018;9:792.
Ozaki KI, Awazu M, Tamiya M, Iwasaki Y, Harada A, Kugisaki S, et al. Targeting the ERK signaling pathway as a potential treatment for insulin resistance and type 2 diabetes. Am J Physiol Endocrinol Metab. 2016;310:E643–E651.
Prusty D, Park BH, Davis KE, Farmer SR. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem. 2002;277:46226–32.
Murtaza M, Khan G, Aftab MF, Afridi SK, Ghaffar S, Ahmed A, et al. Cucurbitacin E reduces obesity and related metabolic dysfunction in mice by targeting JAK-STAT5 signaling pathway. PLoS ONE. 2017;12:e0178910.
Chan PC, Hsiao FC, Chang HM, Wabitsch M, Hsieh PS. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J. 2016;30:2282–97.
Fiorella PD, Giguere V, Napoli JL. Expression of cellular retinoic acid-binding protein (type II) in Escherichia coli. Characterization and comparison to cellular retinoic acid-binding protein (type I). J Biol Chem. 1993;268:21545–52.
Wei LN, Chang L, Hu X. Studies of the type I cellular retinoic acid-binding protein mutants and their biological activities. Mol Cell Biochem. 1999;200:69–76.
Persaud SD, Lin YW, Wu CY, Kagechika H, Wei LN. Cellular retinoic acid binding protein I mediates rapid non-canonical activation of ERK1/2 by all-trans retinoic acid. Cell Signal. 2013;25:19–25.
Persaud SD, Park SW, Ishigami-Yuasa M, Koyano-Nakagawa N, Kagechika H, Wei LN. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation. Sci Rep. 2016;6:22396.
Park SW, Persaud SD, Ogokeh S, Meyers TA, Townsend D, Wei LN. CRABP1 protects the heart from isoproterenol-induced acute and chronic remodeling. J Endocrinol. 2018;236:151–65.
Lin YL, Persaud SD, Nhieu J, Wei LN. Cellular retinoic acid-binding protein 1 modulates stem cell proliferation to affect learning and memory in male mice. Endocrinology. 2017;158:3004–14.
Park SW, Huang WH, Persaud SD, Wei LN. RIP140 in thyroid hormone-repression and chromatin remodeling of Crabp1 gene during adipocyte differentiation. Nucleic Acids Res. 2009;37:7085–94.
Lin YW, Lee B, Liu PS, Wei LN. Receptor-interacting protein 140 orchestrates the dynamics of macrophage M1/M2 polarization. J Innate Immun. 2016;8:97–107.
Lin YW, Montassier E, Knights D, Wei LN. Gut microbiota from metabolic disease-resistant, macrophage-specific RIP140 knockdown mice improves metabolic phenotype and gastrointestinal integrity. Sci Rep. 2016;6:38599.
Lin YW, Liu PS, Adhikari N, Hall JL, Wei LN. RIP140 contributes to foam cell formation and atherosclerosis by regulating cholesterol homeostasis in macrophages. J Mol Cell Cardiol. 2015;79:287–94.
Takahashi M, Kamei Y, Ezaki O. Mest/Peg1 imprinted gene enlarges adipocytes and is a marker of adipocyte size. Am J Physiol Endocrinol Metab. 2005;288:E117–124.
Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, Cawthorn WP, et al. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122:2405–16.
Yen A, Roberson MS, Varvayanis S, Lee AT. Retinoic acid induced mitogen-activated protein (MAP)/extracellular signal-regulated kinase (ERK) kinase-dependent MAP kinase activation needed to elicit HL-60 cell differentiation and growth arrest. Cancer Res. 1998;58:3163–72.
Bost F, Caron L, Marchetti I, Dani C, Le Marchand-Brustel Y, Binetruy B. Retinoic acid activation of the ERK pathway is required for embryonic stem cell commitment into the adipocyte lineage. Biochem J. 2002;361(Pt 3):621–7.
Murray T, Russell TR. Inhibition of adipose conversion in 3T3-L2 cells by retinoic acid. J Supramol Struct. 1980;14:255–66.
Kamei Y, Kawada T, Mizukami J, Sugimoto E. The prevention of adipose differentiation of 3T3-L1 cells caused by retinoic acid is elicited through retinoic acid receptor alpha. Life Sci. 1994;55:PL307–312.
Jeyakumar SM, Vajreswari A, Sesikeran B, Giridharan NV. Vitamin A supplementation induces adipose tissue loss through apoptosis in lean but not in obese rats of the WNIN/Ob strain. J Mol Endocrinol. 2005;35:391–8.
Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–96.
Berry DC, DeSantis D, Soltanian H, Croniger CM, Noy N. Retinoic acid upregulates preadipocyte genes to block adipogenesis and suppress diet-induced obesity. Diabetes. 2012;61:1112–21.
Kim DM, Choi HR, Park A, Shin SM, Bae KH, Lee SC, et al. Retinoic acid inhibits adipogenesis via activation of Wnt signaling pathway in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2013;434:455–9.
Wang B, Fu X, Zhu MJ, Du M. Retinoic acid inhibits white adipogenesis by disrupting GADD45A-mediated Zfp423 DNA demethylation. J Mol Cell Biol. 2017;9:338–49.
Erkelens MN, Mebius RE. Retinoic acid and immune homeostasis: a balancing act. Trends Immunol. 2017;38:168–80.
Karkeni E, Bonnet L, Astier J, Couturier C, Dalifard J, Tourniaire F, et al. All-trans-retinoic acid represses chemokine expression in adipocytes and adipose tissue by inhibiting NF-kappaB signaling. J Nutr Biochem. 2017;42:101–7.
Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, et al. A functionally specialized population of mucosal CD103 + DCs induces Foxp3 + regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–64.
Gundra UM, Girgis NM, Gonzalez MA, San Tang M, Van Der Zande HJP, Lin JD, et al. Vitamin A mediates conversion of monocyte-derived macrophages into tissue-resident macrophages during alternative activation. Nat Immunol. 2017;18:642–53.
Safonova I, Darimont C, Amri EZ, Grimaldi P, Ailhaud G, Reichert U, et al. Retinoids are positive effectors of adipose cell differentiation. Mol Cell Endocrinol. 1994;104:201–11.
Safonova I, Reichert U, Shroot B, Ailhaud G, Grimaldi P. Fatty acids and retinoids act synergistically on adipose cell differentiation. Biochem Biophys Res Commun. 1994;204:498–504.
Berry DC, Soltanian H, Noy N. Repression of cellular retinoic binding protein II during adipocyte differentiation. J Biol Chem. 2010;285:15324–32.
Gorry P, Lufkin T, Dierich A, Rochette-Egly C, Decimo D, Dolle P, et al. The cellular retinoic acid binding protein I is dispensable. Proc Natl Acad Sci USA. 1994;91:9032–6.
de Bruijn DR, Oerlemans F, Hendriks W, Baats E, Ploemacher R, Wieringa B, et al. Normal development, growth and reproduction in cellular retinoic acid binding protein-I (CRABPI) null mutant mice. Differentiation. 1994;58:141–8.
Scherer PE. Adipose tissue: from lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–45.
Mariash CN. Thyroid hormone and the adipocyte. J Clin Endocrinol Metab. 2003;88:5603–4.
Obregon MJ. Adipose tissues and thyroid hormones. Front Physiol. 2014;5:479.
Wei LN, Hu X. Receptor interacting protein 140 as a thyroid hormone-dependent, negative co-regulator for the induction of cellular retinoic acid binding protein I gene. Mol Cell Endocrinol. 2004;218:39–48.
Aubert J, Dessolin S, Belmonte N, Li M, McKenzie FR, Staccini L, et al. Leukemia inhibitory factor and its receptor promote adipocyte differentiation via the mitogen-activated protein kinase cascade. J Biol Chem. 1999;274:24965–72.
Belmonte N, Phillips BW, Massiera F, Villageois P, Wdziekonski B, Saint-Marc P, et al. Activation of extracellular signal-regulated kinases and CREB/ATF-1 mediate the expression of CCAAT/enhancer binding proteins beta and -delta in preadipocytes. Mol Endocrinol. 2001;15:2037–49.
Bost F, Aouadi M, Caron L, Even P, Belmonte N, Prot M, et al. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–11.
Tang QQ, Otto TC, Lane MD. Mitotic clonal expansion: a synchronous process required for adipogenesis. Proc Natl Acad Sci USA. 2003;100:44–49.
Acknowledgements
The authors thank Neal Mukherjee for technical support.
Funding
This work was supported by NIH grants DK54733, DK60521, and the Dean’s Commitment and the Distinguished McKnight Professorship of University of Minnesota to LNW.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Lin, YW., Park, S.W., Lin, YL. et al. Cellular retinoic acid binding protein 1 protects mice from high-fat diet-induced obesity by decreasing adipocyte hypertrophy. Int J Obes 44, 466–474 (2020). https://doi.org/10.1038/s41366-019-0379-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-019-0379-z
This article is cited by
-
Modulation of adipose inflammation by cellular retinoic acid-binding protein 1
International Journal of Obesity (2022)
-
Regulation of exosome secretion by cellular retinoic acid binding protein 1 contributes to systemic anti-inflammation
Cell Communication and Signaling (2021)