Abstract
Dynamic interactions between organelles are responsible for a variety of intercellular functions, and the endoplasmic reticulum (ER)–mitochondrial axis is recognized as a representative interorganelle system. Several studies have confirmed that most proteins in the physically tethered sites between the ER and mitochondria, called mitochondria-associated ER membranes (MAMs), are vital for intracellular physiology. MAM proteins are involved in the regulation of calcium homeostasis, lipid metabolism, and mitochondrial dynamics and are associated with processes related to intracellular stress conditions, such as oxidative stress and unfolded protein responses. Accumulating evidence has shown that, owing to their extensive involvement in cellular homeostasis, alterations in the ER–mitochondrial axis are one of the etiological factors of tumors. An in-depth understanding of MAM proteins and their impact on cell physiology, particularly in cancers, may help elucidate their potential as diagnostic and therapeutic targets for cancers. For example, the modulation of MAM proteins is utilized not only to target diverse intracellular signaling pathways within cancer cells but also to increase the sensitivity of cancer cells to anticancer reagents and regulate immune cell activities. Therefore, the current review summarizes and discusses recent advances in research on the functional roles of MAM proteins and their characteristics in cancers from a diagnostic perspective. Additionally, this review provides insights into diverse therapeutic strategies that target MAM proteins in various cancer types.
Similar content being viewed by others
Introduction
An understanding of the cooperation between organelles is crucial for revealing the mechanisms that modulate cellular functions and homeostasis. Among interorganellar networks, the connection between the endoplasmic reticulum (ER) and mitochondria has been extensively studied owing to its diverse functions and impact on the pathogenesis of multiple diseases. The concept of a functional unit comprising the ER and mitochondria was first proposed in 19501. The adjacent membrane sites that physically tether the ER and mitochondria are called mitochondria-associated ER membranes (MAMs); technological advances in microscopy have enabled the elucidation of the physiological features of the tethering structures of the MAMs. The ER and mitochondria are separated by a 6–15 nm gap, and the average surface area percentage of mitochondria covered by MAMs was calculated to be 3–5% in mammalian cells2.
MAMs represent an etiological and therapeutic target in cardiovascular diseases3, neurodegenerative diseases4, metabolic disorders5,6, and cancers. In this review, we discuss the associations between alterations in MAM proteins and cancers and present recent advances in research on these associations. Additionally, we discuss the contribution of MAM proteins to tumorigenesis and cancer progression as well as their possible applications as diagnostic and therapeutic targets.
Structure and functional role of ER–mitochondria contact sites
Calcium regulation
Maintenance of Ca2+ homeostasis is one of the most important functions of MAMs, as the ER functions as the main regulator and storage organelle of calcium ions within living cells7. The resting levels of Ca2+ in mitochondria are similar to those in the cytosol; however, they can increase to 100 times the cytosolic levels under specific stimulation conditions8. A contributing factor to this drastic increase has been identified and subsequently confirmed by the Ca2+ microdomain hypothesis, which states that the outer membrane of mitochondria contains hotspots for Ca2+ shuttling from the ER9,10,11. As the affinity of the mitochondrial calcium uniporter (MCU) located in the inner mitochondrial membrane is dependent on the local Ca2+ concentration, these microdomains facilitate Ca2+ influx through the MCU12,13.
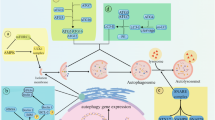
The translocation of Ca2+ in MAMs is mediated by several proteins. The inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) is a representative Ca2+ channel located in the ER14 (Fig. 1). The opening of this receptor and subsequent Ca2+ transport occur when the binding site of each tetrameric subunit of IP3R is concatenated with IP315. The IP3 binding affinity and Ca2+ influx activity of IP3R vary depending on its subtype16, phosphorylation17, and interactions with other regulatory proteins. Additionally, recent research has shown that the localization of mobile IP3R on MAMs is important for Ca2+ signaling between the ER and mitochondria18.
The figure shows representative MAM proteins and their mechanisms of regulation of multiple cellular functions. (1) Calcium regulation is modulated mainly by the IP3R-GRP75-VDAC-MCU and VAPB-PTPIP51 complexes in MAMs. (2) Lipid synthesis and transfer are mediated by the enzymes PSS1/2, PSD, and PEMT and the Mdm12-Mmm1-Mdm34-Mdm10 complex. (3) Drp1 and MFN1/2 are involved in mitochondrial fission and fusion, respectively. The PINK1/Parkin pathway mediates mitophagy. ER endoplasmic reticulum, OMM outer mitochondrial membrane, IMM inner mitochondrial membrane. IP3R inositol 1,4,5-triphosphate receptor, GRP75 glucose-related regulated protein 75, VDAC voltage-dependent anion channel, MCU mitochondria calcium uniporter, VAPB vesicle-associated membrane protein B, PTPIP51 protein tyrosine phosphatase-interacting protein-51, PS phosphatidylserine, PE phosphatidylethanolamine, PC phosphatidylcholine, PSS1/2 phosphatidylserine synthase 1/2, PSD phosphatidylserine decarboxylase, PEMT phosphatidylethanolamine-N-methyltransferase, MFN1/2 mitofusin 1/2, PINK1 PTEN-induced putative kinase 1, TOM mitochondrial translocase of the outer membrane 70.
The canonical microdomain of the Ca2+ regulator in MAMs consists of IP3R located in the ER, voltage-dependent anion channel 1 (VDAC1) in the outer mitochondrial membrane (OMM), and glucose-regulated protein 75 (GRP75), which acts as a physical link between IP3R and VDAC1 and directly affects mitochondrial Ca2+ accumulation19. The formation of these complexes brings the ER and mitochondria into close proximity, resulting in the formation of microdomains with high Ca2+ levels20,21. Recent evidence has indicated the importance of another protein component within this microdomain. DJ-1 was recognized as the fourth component of the MAM complex through the observation that DJ-1 ablation induced IP3R3 aggregation, which prevented the tethering of the IP3R-GRP75-VDAC microdomain22.
In addition to the IP3R-GRP75-VDAC1 complex, the interaction of ER-integrated protein vesicle-associated membrane protein B (VAPB) with the OMM protein called protein tyrosine phosphatase-interacting protein-51 (PTPIP51) is also involved in Ca2+ regulation. Depletion of either VAPB or PTPIP51 leads to the disruption of MAMs and perturbation of Ca2+ transport23.
Lipid metabolism
Because lipid synthesis is compartmentalized, lipids must be transferred between organelle compartments. Lipids shuttle between specific organelles through vesicle trafficking; however, lipid influx into mitochondria through vesicles is not possible even when lipids are needed24. Thus, several MAM proteins regulate the nonvesicular trafficking of lipids from the ER to mitochondria (Fig. 1). Phosphatidylserine synthase 1/2 (PSS1/2) is a representative synthetic enzyme that is enriched in MAMs and mediates phosphatidylserine (PS) synthesis25. Specifically, PSS1 and PSS2 convert phosphatidylcholine (PC) and phosphatidylethanolamine (PE), respectively, into PS. PE import relies on the conversion of transported PS in MAMs to PE by PS decarboxylase (PSD) in mitochondria rather than direct import26. Disruption of this process and the mitochondrial PE level impairs mitochondrial dynamics and bioenergetics27,28. Mitochondrial PE can be traced back to MAMs and is converted into PC by PE-N-methyltransferase29. This transfer system is the rate-limiting step in lipid biogenesis and further contributes to the maintenance of phospholipid homeostasis.
The complex consisting of Mdm10 and Mdm34 is located in the OMM, and Mmm1 in the ER and Mdm12 in the cytosol exhibit features of ER–mitochondria tethering proteins and phospholipid exchangers30. Mdm34, Mmm1, and Mdm12 physically interact with phospholipids via their synaptotagmin-like mitochondrial lipid-binding domains31,32,33. The direct binding of Mmm1 and Mdm12 forms a hydrophobic cavity that mediates the transport of glycerophospholipids except for PE34. However, as the depletion of this complex only exerts minor effects on the lipidome, more unknown lipid regulatory proteins and mechanisms may exist in MAMs30.
Regulation of mitochondrial dynamics
Mitochondrial quality control is a defense mechanism against mitochondrial insult. In the early stages of quality control, translocation and recruitment of dynamin-related protein (DRP1) in mitochondria occurs in MAMs and facilitates mitochondrial fission35. In contrast, mitofusin 1 (MFN1), another MAM protein, forms puncta in the ER and facilitates mitochondrial fusion36. Physical tethering of the ER to mitochondria by MFN1/2 indicates the importance of MAMs as key sites for regulating mitochondrial dynamics37,38 (Fig. 1).
In addition to fission and fusion, self-degradation of mitochondria upon severe injury, a process called mitophagy, is also influenced by MAM proteins. The PTEN-induced putative kinase (PINK)/parkin pathway is the main signaling pathway for mitophagy. PINK is degraded by mitochondria-resident enzymes and further degraded in lysosomes under normal conditions; however, mitochondrial dysfunction leads to the formation of uncleaved PINK and its accumulation in the OMM through an interaction with TOM39. The accumulated PINK proteins recruit parkin, which induces mitophagy through its E3 ligase activity39,40. A recent study reported that PINK1/Parkin mediate MFN2 phosphorylation, resulting in the dissociation of the MFN2 complex via the p97-dependent pathway. This indicates a relationship between a decrease in ER–mitochondrial contact and mitophagy41. Additionally, assembly of the autophagosome marker ATG14 occurs in MAMs under starvation conditions, and disruption of the ER–mitochondria interaction inhibits ATG14 localization and autophagosome formation42.
Interaction between the ER–mitochondrial axis and calcium homeostasis
Most ER proteins are involved in regulating Ca2+ homeostasis43. For instance, the sigma-1 receptor (Sig-1R), a MAM protein, is enriched in the ER vesicles involved in this process. In the resting state, Sig-1R binds to another chaperone in the ER, GRP78. However, this complex dissociates under ER stress conditions, including Ca2+ depletion. The dissociated Sig-1R then binds to IP3R, mediating its stabilization and Ca2+ influx44. PDZ domain-containing protein 8 (PDZD8) in the ER is another example of a Ca2+-regulating protein in MAMs. PDZD8 knockdown impairs ER–mitochondria tethering and further inhibits mitochondrial Ca2+ uptake in the MAMs of mammalian neurons45. Other proteins modulate calcium homeostasis in MAMs, as shown in Table 1.
As Ca2+ participates in diverse cellular processes, disrupted homeostasis and improper regulation of Ca2+ dynamics in MAMs can negatively affect cellular function. The influx of Ca2+ into mitochondria is essential for bioenergetics because several intramitochondrial enzymes associated with glycolysis and the tricarboxylic acid cycle are activated in a calcium-dependent manner46. The lack of constitutive Ca2+ influx through IP3 reduces the enzymatic activity of pyruvate dehydrogenase and thus the production of adenosine triphosphate (ATP), resulting in the activation of autophagy via the AMPK pathway47. Translocase of mitochondrial outer membrane 70 (TOM70) also affects constitutive Ca2+ shuttling by mediating the linkage between IP3R3 and VDAC, and the depletion of TOM70 results in impaired mitochondrial respiration48.
MAM proteins stimulate Ca2+-dependent apoptotic pathways. Ca2+ overload in the mitochondrial matrix increases mitochondrial permeability by opening mitochondrial permeability transition pores (mPTPs)49. One mechanism of permeability transition is Ca2+-inducible conformational alteration of F-ATP synthases that bind to and show activity toward mPTPs50. The opening of mPTPs disrupts the osmotic balance in mitochondria due to nonselective permeabilization, resulting in an influx of water that induces the release of caspase cofactors51. Furthermore, alterations in Ca2+ levels are closely associated with responses to multiple intracellular stresses, such as ER and oxidative stress.
ER–mitochondria contacts modulate oxidative stress
Oxidative stress results from an imbalance between the production and accumulation of reactive oxygen species (ROS) in cells and is a hallmark of the ability to detoxify or repair reactive products52. ROS are produced primarily in mitochondria and play important roles in cell growth, differentiation, and death53,54. Although low levels of ROS play an essential role in intracellular signaling and pathogen defense, elevated levels can have detrimental effects on cells, such as decreasing the efficiency of mitochondrial respiration and inducing oncogenic stress55. Imbalances in ROS accumulation can contribute to the development and progression of several diseases, including cancer, metabolic disorders, diabetes, and cardiovascular diseases56,57.
The ER and mitochondrial axes play essential roles in the detection of and response to stress conditions, including oxidative stress, and form interconnected networks58. Furthermore, the simultaneous induction of ER stress and overproduction of ROS in several diseases highlights the importance of this axis59. The roles of ROS-related MAM proteins, including endoplasmic reticulum oxidoreductase 1 (ERO1), Sig-1R, p66Shc, and MFN2, have been reported (Fig. 2).
The figure shows the regulation of reactive oxygen species (ROS) production by several proteins present in the mitochondrial ER membrane. Reduced ERO1 generates ROS through an interaction with FAD. ERO1a, one of the ERO1 isoforms, regulates ROS production through an interaction with IP3R by releasing calcium ions into mitochondria, which induces chronic ER stress. Additionally, p66Shc modulates ROS production via phosphorylation at Ser36, Ser54, and Thr38 by ERK, JNK, and P38. Finally, MFN2 regulates ROS production in a manner dependent on its expression level through an interaction with PERK. ERO1 endoplasmic reticulum oxidoreductase 1, ERp44 endoplasmic reticulum protein 44, IP3R inositol 1,4,5-triphosphate receptor, GRP75 glucose-related regulated protein 75, VDAC voltage-dependent anion channel, CytoC cytochrome c, FAD flavin adenine dinucleotide, PERK protein kinase R-like endoplasmic reticulum kinase.
ERO1 is located entirely on the MAMs close to the ER surface and is an essential factor in the ER oxidative folding mechanism through co-localization with protein disulfide isomerase (PDI)60. PDI catalyzes the formation of disulfide bonds in unfolded proteins during oxidative protein folding and is then converted to a reduced form61. Reduced PDI is subsequently oxidized by ERO1 to participate in the catalytic reaction cycle, where reduced ERO1 transfers electrons to an oxygen molecule via flavin adenine dinucleotide, releasing H2O260. ERO1α, an ERO1 isoform, is overexpressed in various cancers, and its expression is increased by chronic ER stress, resulting in excessive H2O2 production and an increased ROS burden62. ERO1 also affects ROS production by regulating other MAM proteins. Under stress conditions, ERO1 oxidizes IP3R1 and induces detachment of the disulfide isomerase–like protein ERp44 from IP3R163. ERp44 has an inhibitory effect on IP3R164, leading to massive influx of Ca2+ through IP3R, which ultimately results in upregulated mitochondrial metabolism and excessive ROS production65,66.
Sig-1R regulates Ca2+ homeostasis and is involved in ROS-related signaling pathways. Although the ROS-regulatory mechanisms of Sig-1R are not fully understood, previous studies have shown that Sig-1R knockdown leads to ROS accumulation67,68. Furthermore, some Sig-1R agonists exhibit antioxidant activity under pathological conditions69.
p66Shc is located in MAMs, mitochondria, and the cytosol and tetramerizes in response to oxidative stress70. Under oxidative stress conditions, its Ser36 residue is phosphorylated by p38MAPK, ERK, and JNK1/2, and phosphorylation of other residues, namely, Ser54 and Thr386, occurs to prevent p66Shc degradation by ubiquitination71,72,73. Activated p66Shc translocates through MAMs into mitochondria, where it binds to cytochrome c to generate ROS and ultimately induce cell death74. The generation of ROS by activated p66Shc is supported by previous studies showing that both p66Shc knockout mice and cells exhibit reduced oxidative stress levels and a decreased incidence of diseases such as atherosclerosis75,76.
As previously described, both MFN1 and MFN2 are involved in the promotion of mitochondrial fusion. However, the fusion process, which relies primarily on MFN1 and MFN2, is speculated to have additional distinct functions77. The possible effects of MFN2 on ROS generation have been suggested to be due to other functions of MFN2. Munoz et al.78 reported the possible inhibitory effects of MFN2 on ROS production. MFN2 directly interacts with an ER stress branch, the pancreatic endoplasmic reticulum kinase (PERK) pathway, and inhibits ER stress pathways and ROS production. Other studies have shown that MFN2 overexpression activates the PERK/activating transcription factor 4 (ATF4) pathway and reduces ROS levels in cardiac fibroblasts79. However, a recent study showed that MFN2 facilitates the adaptation of macrophages to mitochondrial respiration and ROS generation in response to inflammatory stimuli80. Thus, further research is required to fully understand the different functions of MFN2 in different cell types and under specific stress conditions.
Interaction between ER stress and the ER–mitochondria axis
Protein folding is the main function of the ER. Various conditions, such as disruption of Ca2+ homeostasis, inhibition of degradation of unfolded proteins due to proteasome blockade, and genetic mutations, can cause the accumulation of unfolded proteins81. Under these stress conditions, the unfolded protein response (UPR) is activated by three ER transmembrane proteins: activating transcription factor 6 (ATF6), inositol-requiring enzyme 1α (IRE1α), and PERK82. Under normal conditions, the ER chaperone GRP78/BiP binds to the ER lumen region of these transmembrane proteins and inhibits their activity. However, under stress conditions, GRP78 binds to misfolded proteins and induces the activation of these three transmembrane proteins83.
In the ATF6 pathway of the ER stress response, sensors mediate the UPR, and ATF6 translocates to the Golgi complex after GRP78 is released. ATF6 is first cleaved by site-1 protease, and one half remains at the NH2-terminus before being cleaved by site-2 protease84. Regarding the IRE pathway, GRP78 is normally bound to IRE1α or its homolog, IRE1p, and maintains its inactivation. When GRP78 dissociates from IRE1 in ER-stressed cells, IRE1 is phosphorylated and dimerizes85. Finally, activated PERK phosphorylates eIF2α and further increases the translation of selected mRNAs, including ATF4, which then promotes the expression of transcription factors, such as C/EBP homologous protein (CHOP), leading to growth arrest and DNA damage86. CHOP overexpression causes apoptosis by translocating B-cell lymphoma 2 (BCL2)-associated X (a proapoptotic protein) to mitochondria and decreasing the expression of BCL2 (an antiapoptotic protein)87.
The associations between MAM components and ER stress have been widely reported (Table 2), and some UPR-related proteins also function as MAM components. The interaction between PERK and MFN2 is a representative example of the UPR-related MAM pathway. Additionally, some MAM proteins are regulated by ER stress; for instance, Sig-1R is transcriptionally upregulated via the PERK/eIF2α/ATF4 pathway88, while another MAM protein, Rab32, is upregulated via the UPR pathway. Rab32 belongs to the Ras-like small GTPase family and is involved in mitochondrial fission via interaction with DRP189. In SH-SY5Y cells, Rab32 expression is elevated upon induction of ER stress (thapsigargin treatment), leading to mitochondrial dysfunction and neuronal death90. Furthermore, the ER chaperone GRP78 binds to IP3R1 during the ER stress response, releasing Ca2+ for influx into mitochondria and inducing cell death due to mitochondrial dysfunction91.
Further evidence has also revealed that several MAM proteins affect UPR pathways. The ER protein VAPB is an important protein involved in UPR activity, and VABP loss inhibits IRE1/XBP1 activity in response to ER stress92. Furthermore, VAPB interacts with ATF6 in response to ER stress, and the terminal domain of ATF6 senses protein accumulation in the ER lumen. VAPB, with no luminal structure, is not directly regulated by ATF6 activation but is indirectly inhibited93. VAPB-induced ER stress has been implicated in inducing mitochondrial dysfunction by releasing Ca2+ through interactions with PTPIP51 in the mitochondrial membrane23.
Characteristics and diagnostic role of ER–mitochondria contact sites in cancers
Cancer cells require a substantial amount of energy for their rapid proliferation and acquisition of malignant phenotypes and use various methods, such as increases in glucose uptake and glycolytic activity (a phenomenon known as the Warburg effect), lipid synthesis and lipolysis, and modulation of Ca2+ signaling, to meet these requirements94,95,96. Therefore, MAMs play important roles in cancer cell function and metabolism, as they regulate the aforementioned pathways (Fig. 3).
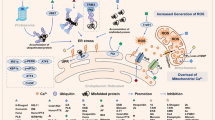
The figure shows representative alterations in MAMs in cancer cells from three perspectives (Ca2+ signaling, mitophagy, and lipid metabolism) and the therapeutic drugs that target them. In cancer, the function of the IP3R-GRP75-VDAC complex is impaired, thus limiting Ca2+ trafficking to mitochondria and inducing resistance to mitochondrial apoptosis. Cisplatin targets IP3R and promotes the activity of its complex, which activates the influx of Ca2+ into mitochondria and induces apoptosis. Additionally, p53 mutations have been detected in various cancers, and these mutations result in the inhibition of Ca2+ influx into the ER and thus in cell death. Adriamycin increases p53 levels in MAMs and facilitates Ca2+ influx into the ER through SERCA, promoting apoptosis in cancer. Mipsagargin inhibits SERCA activity and increases the intracellular Ca2+ level, which can trigger cancer cell death. Resveratrol promotes Ca2+ signaling through IP3R, resulting in autophagy-induced cancer cell death. ACAT-1 generates cholesteryl esters that induce the accumulation of lipid droplets, resulting in tumor growth and metastasis. Mitotane inhibits ACAT-1 and causes free cholesterol accumulation in the ER, leading to ER stress-mediated apoptosis in cancer cells.
The regulation of Ca2+ signaling is crucial in cancers, as it is involved in cancer progression, epithelial-to-mesenchymal transition, invasion, and resistance to apoptosis97. Therefore, Ca2+-regulating proteins in MAMs play various roles in cancer development (Table 3). The IP3R-GRP75-VDAC-MCU complex, which plays an important role in Ca2+ transport, is regulated by oncoproteins such as PTEN, BRCA1, and BCL298. In MAMs, PTEN binds to IP3R and prevents its degradation, thereby promoting Ca2+ transport to mitochondria, which is important for apoptosis98,99. However, in various cancers, PTEN loses functionality and triggers inappropriate Ca2+ transport, leading to apoptosis resistance100,101. BCL2, another oncoprotein in MAMs, interacts with IP3R and VDAC and prevents the translocation of Ca2+ from the ER to mitochondria. Furthermore, the interaction between BCL2 and VDAC1 interferes with the export of cytochrome c from mitochondria and thus hinders apoptosis98,102. Therefore, BCL2 overexpression in cancers results in resistance to apoptosis.
BRCA1-associated protein 1 (BAP1), a tumor suppressor protein in MAMs, facilitates Ca2+ influx into mitochondria by interacting with IP3R103. Abnormalities in the function of BAP1 can induce inappropriate Ca2+ influx into mitochondria, which may affect the regulation of apoptosis and lead to carcinogenesis104. Mutations in BAP1 have been observed in various cancers, including renal cell carcinoma, cutaneous melanoma, and uveal melanoma104. GRP75 also plays an important role in the regulation of Ca2+ homeostasis2. Transglutaminase type 2 modulates GRP75 function by binding to GRP75 and increasing Ca2+ flux between the ER and mitochondria, which affects cancer growth and metastasis. Upregulation of transglutaminase type 2 is a hallmark of breast cancer105,106. Additionally, TOM70, a protein that links IP3R3 to VDAC, exhibits notably high expression levels in breast cancer cells, and its potential as a therapeutic target has been duly recognized in previous studies48,107.
In addition to its proapoptotic role in mitochondria, Ca2+ is important for energy production, progression, and metastasis in cancer108,109. Ca2+ influx into mitochondria mediated by MCU promotes mitochondrial biogenesis and colon cancer proliferation108, and impairment of Ca2+ uptake by MCU knockdown inhibits the proliferation of embryonal rhabdomyosarcoma110. Other types of cancers with high MCU expression include prostate, ovarian, and breast cancers, indicating the diagnostic utility of MCU expression in cancer111. Moreover, PDZD8, another Ca2+-regulating protein in MAMs, was found to exhibit increased expression levels in stomach cancer tissue compared with normal tissue and is involved in the proliferation and metastasis of stomach cancer112.
Although research on the exact role of ROS in cancers is still underway, ROS are known to be involved in cancer progression and metastasis98. Several MAM proteins, including p66hsc, are regulated by ROS, and p66hsc and the oncoprotein p53 regulate each other113. Furthermore, p66hsc can be activated by steroid hormones, and activated p66hsc interacts with cytochrome c to increase ROS production. These alterations, including oxidative stress, have been reported to result in poor prognosis in patients with prostate cancer72,114,115,116. These characteristics of p66hsc have also been observed in other cancers, including breast and lung cancers, indicating its potential as a diagnostic and therapeutic target117,118,119. Furthermore, ERO1, which controls ROS production through the regulation of MAM proteins, is overexpressed in cholangiocarcinoma and is involved in proliferation and metastasis, leading to poor prognosis in patients120. Notably, ERO1 is also overexpressed in various other cancers, including breast cancer, lung cancer, and hepatocellular carcinoma, in which it ultimately results in poor prognosis121,122,123.
Activated lipid metabolism and the accumulation of lipid droplets are hallmarks of various cancer cells95. Elevated lipid levels in cancer cells promote proliferation and serve as energy reserves and messengers in oncogenic pathways95,124. Furthermore, various enzymes involved in lipid synthesis are upregulated in various cancers, including lung, ovarian, and prostate cancers95,125,126. Various enzymes involved in lipid synthesis, such as fatty acid CoA ligase, which catalyzes the ligation of triacylglycerols and ceramide, and acyl-coenzyme A:cholesterol acyltransferase-1 (ACAT-1), which catalyzes the synthesis of cholesterol, are mainly located in MAMs98,127,128. Therefore, alterations in the expression of these enzymes in MAMs are strongly associated with cancer. For example, after passing through mitochondria, ceramide plays an important role as an apoptosis inducer and can inhibit cancer growth and cell death129,130. Cholesterol metabolism is strongly associated with cancer. ACAT-1 in MAMs converts cholesterol to cholesteryl esters, which accumulate in the lipid droplets of cancer cells98,131. These accumulated cholesteryl esters have a considerable impact on the proliferation and metastasis of cancer cells132. Caveolin-1, located in MAMs, is involved in cholesterol efflux, and its overexpression has been identified in a variety of cancers, such as lung, liver, kidney, and colon cancers133. These expression patterns of caveolin-1 are closely related to cancer progression, metastasis, and drug resistance134,135,136. Therefore, various MAM proteins play major roles in cancer and can potentially be used in diagnosis and treatment. The association between ER stress and cancer has been established137. Sig-1R, a MAM protein regulated by ER stress, has been reported to be overexpressed in myelogenous leukemia and colon cancer138. This increased expression promotes angiogenesis and facilitates cancer cell migration, resulting in poor prognosis in patients. Consequently, Sig-1R is considered a promising therapeutic target138. Another MAM protein associated with ER stress, VAP-B, has been reported to play a key role in breast cancer progression, highlighting its potential as a diagnostic marker for this malignancy139.
The ER–mitochondrial axis as a therapeutic target
Targeting Ca2+ signaling
The characteristic functions of MAMs, including those in Ca2+ and ROS signaling, lipid metabolism, autophagy, and mitochondrial fission, enable their use as diagnostic markers and therapeutic targets for cancer (Fig. 3). Different methods can be used to trigger cancer cell apoptosis by promoting Ca2+ transport through modulation of MAM proteins. One of the most widely used anticancer drugs, cisplatin, is used to treat various cancers, including ovarian, breast, lung, and bladder cancers140. In ovarian cancer (SKOV3) cells, cisplatin promotes Ca2+ translocation from the ER to mitochondria and cytosol, causing ER stress-mediated apoptosis141. Other cancer therapeutics, such as adriamycin and mipsagargin, target Ca2+ signaling. In MAMs, p53 regulates the activity of SERCA by binding to it, leading to Ca2+ influx into the ER and resulting in increased apoptosis142. p53 mutations have been detected in various types of cancers, and adriamycin can increase p53 levels in MAMs, which promotes Ca2+ signaling and apoptosis in cancer cells through the activation of SERCA111,142,143. Mipsagargin inhibits SERCA function, resulting in an increase in intracellular Ca2+, which induces apoptosis in cancer cells144. Another component of the Ca2+ transport complex, VDAC, can potentially serve as a biomarker and therapeutic target for breast cancer, as its overexpression was detected in a previous study145. Furthermore, VDAC1 inhibition by siRNA induces cancer cell apoptosis, suggesting that siRNAs could be a target for cancer therapy146,147. Previous studies have shown that PDZD8, which is highly expressed in stomach cancer and is involved in cancer cell proliferation and metastasis, can also be used as a therapeutic target112. Notably, sunitinib, a kinase inhibitor, attenuates the proliferation of stomach cancer cells, as demonstrated in the human gastric cancer cell lines TMK1 and MKN74, by decreasing the PDZD8 protein level112.
Targeting lipid metabolism and ER stress
Targeting the lipid metabolism-related functions of MAMs could aid in cancer treatment. For example, mitotane, which targets ACAT-1, converts cholesterol to CE and causes lipid droplet formation in various cancers148. In adrenocortical carcinoma, mitotane-induced ACAT-1 suppression induces free cholesterol and fatty acid accumulation in the ER, leading to apoptosis111,148,149. Modulation of ER stress also constitutes a potential therapeutic approach for cancer. In prostate cancer, corosolic acid modulates IRE1 and PERK signaling and induces ER stress, which promotes apoptosis and inhibits cell proliferation150. In hepatocarcinoma, 20(S)-protopanaxadiol can increase UPR activity and enhance the ER stress response by phosphorylating components of the PERK cascade, subsequently leading to increases in the expression of associated genes151. Moreover, previous studies have shown that panaxydol induces Ca2+ release from the ER through IP3R and activates the JNK pathway, causing ER stress, which is mediated by PERK152,153. These effects trigger apoptosis in renal carcinoma and prostate cancer cells152,153. Evodiamine is another therapeutic candidate that affects the JNK and PERK pathways. By modulating both pathways, evodiamine can induce apoptosis in ovarian cancer cells and reduce the extent of metastasis in colon cancer153,154,155.
Increasing the sensitivity of cancer cells to chemotherapeutic compounds
Repeated use of chemotherapeutic drugs can result in resistance to them; therefore, other MAM proteins can be targeted to reduce this resistance. For example, cisplatin is widely used to treat ovarian cancer; however, its long-term use can induce cisplatin resistance in ovarian cancer cells, which is highly correlated with GRP75156. GRP75 knockdown increases cisplatin-induced apoptosis in ovarian cancer cells, suggesting a decrease in resistance157,158. Blocking the function of MAM-localized BCL2, which interacts with IP3R, via a BCL2 inhibitor disrupts Ca2+ translocation and leads to an increase in the cellular Ca2+ level in cisplatin-resistant ovarian cancer cells. Furthermore, a study demonstrated that ABT737, a BCL2 inhibitor, reduces the cisplatin resistance of SKOV3 ovarian cancer cells by modulating Ca2+ signaling159,160. These changes in cellular Ca2+ signaling lead to cisplatin-induced apoptosis, indicating that the regulation of MAM proteins could lower resistance to anticancer agents159,160. Targeting MAM-localized PERK is also a potential approach for treating resistant forms of cancers. PERK regulates ER stress, ROS production, and Ca2+ levels, all of which affect the apoptotic process59,161. Previous studies have reported that modulating PERK can induce apoptosis in endocrine-resistant breast cancer cells161,162,163. Another example of increased anticancer treatment sensitivity is that occurring after combination treatment with bortezomib, a proteasome inhibitor, and cisplatin. In pancreatic cancer, bortezomib can maximize the anticancer effects of cisplatin by activating JNK cascades and subsequently inducing apoptosis159,164.
Reducing cancer metastasis
Emerging evidence suggests that MAM proteins and mitochondrial calcium dynamics may affect the migratory ability of cells165, and several studies have revealed the roles of MAM proteins in tumor invasion and metastasis, offering valuable perspectives for both diagnostic and therapeutic approaches. In triple-negative breast cancer cells, blocking MCU function can inhibit Ca2+ influx into mitochondria and ROS generation, resulting in reduced migration and progression159,166. Moreover, overexpression of FUN14 domain-containing 1 (FUNDC1), a MAM protein, could be a diagnostic and prognostic marker for breast cancer, as it triggers cell proliferation, migration, and invasion167. FUNDC1 knockdown by siRNA alters NFATC1 activity and inhibits the proliferation and metastasis of breast cancer cells147,168. A similar example is the targeting of MFN2 by miR-761 in hepatocellular carcinoma discovered in a previous study165, wherein miR-761 was shown to be upregulated in the tissues of patients with hepatocellular carcinoma, thereby confirming its role in modulating MFN2 expression. Additionally, miR-761 inhibition resulted in reduced migration and invasion of human cancer cell lines, as well as suppression of tumor metastasis in nude mice165.
Increasing immune cell activity
Modulation of MAMs may aid in cancer treatment by increasing the accessibility of immune cells. For example, interactions between the ER and mitochondria regulate the expression of glycans, which can reduce immune cell accessibility in glioblastoma169. A previous study proposed the modification of glycan expression in glioblastoma through modulation of ER–mitochondria contact sites to enhance immune cell recognition as a potential approach for glioblastoma treatment169. Furthermore, regulation of MAM proteins in immune and cancer cells can aid in treatment. In memory T cells, promoting AKT signaling can inhibit the expression of MAM-localized GSK3b and strengthen the interaction between VDAC and HK-1, resulting in increased cellular respiration and functional acquisition170. These alterations play a significant role in the differentiation of memory T cells into effector T cells170. These studies suggest that the modulation of MAM proteins to increase immune cell activity offers various therapeutic benefits.
Conclusion
Interactions between organelles are involved in many cellular functions. This review focuses specifically on the contact sites between the ER and mitochondria, known as MAMs. Various MAM proteins play important roles in the regulation of Ca2+ signaling, lipid metabolism, mitochondrial dynamics, oxidative stress, and ER stress. Therefore, alterations in MAM proteins can lead to changes in these mechanisms, resulting in the inhibition of apoptosis and increased resistance to anticancer drugs. Several therapeutic agents targeting MAM proteins have been reported to induce apoptosis and reduce antibiotic resistance and metastasis in cancer cells by modulating Ca2+ signaling and lipid metabolism. Owing to these diverse effects in cancers, research on MAM-targeting therapeutics should be ongoing. Moreover, as alterations in MAM proteins are characteristic of various cancers, they can potentially serve as diagnostic markers and therapeutic targets; however, further research is needed to determine whether they can be used as accurate biomarkers for specific cancers.
References
Bernhard, W. & Rouiller, C. Close topographical relationship between mitochondria and ergastoplasm of liver cells in a definite phase of cellular activity. J. Biophys. Biochem. Cytol. 2, 73–78 (1956).
Phillips, M. J. & Voeltz, G. K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol. 17, 69–82 (2016).
Zhang, Y., Yao, J., Zhang, M., Wang, Y. & Shi, X. Mitochondria-associated endoplasmic reticulum membranes (MAMs): possible therapeutic targets in heart failure. 10, https://doi.org/10.3389/fcvm.2023.1083935 (2023).
Liu, J. & Yang, J. Mitochondria-associated membranes: a hub for neurodegenerative diseases. Biomed. Pharmacother. 149, 112890 (2022).
Cheng, H. et al. The molecular mechanisms underlying mitochondria-associated endoplasmic reticulum membrane-induced insulin resistance. 11, https://doi.org/10.3389/fendo.2020.592129 (2020).
López-Crisosto, C. et al. ER-to-mitochondria miscommunication and metabolic diseases. Biochim. Biophys. Acta Mol. Basis Dis. 1852, 2096–2105 (2015).
Meldolesi, J. & Pozzan, T. The endoplasmic reticulum Ca2+ store: a view from the lumen. Trends Biochem. Sci. 23, 10–14 (1998).
Rizzuto, R., Duchen, M. R. & Pozzan, T. Flirting in little space: the ER/mitochondria Ca2+ liaison. Sci. STKE 2004, re1 (2004).
Giacomello, M. et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 38, 280–290 (2010).
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993).
Rizzuto, R. et al. Close contact with the endoplasmic reticulum as determinant of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998).
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011).
Rapizzi, E. et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 159, 613–624 (2002).
Camello, C., Lomax, R., Petersen, O. H. & Tepikin, A. V. Calcium leak from intracellular stores-the enigma of calcium signalling. Cell Calcium 32, 355–361 (2002).
Alzayady, K. J. et al. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal. 9, ra35 (2016).
Miyakawa, T. et al. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J. 18, 1303–1308 (1999).
Vanderheyden, V. et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta 1793, 959–970 (2009).
Katona, M. et al. Capture at the ER-mitochondrial contacts licenses IP(3) receptors to stimulate local Ca(2+) transfer and oxidative metabolism. Nat. Commun. 13, 6779 (2022).
Szabadkai, G. et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911 (2006).
Csordas, G. et al. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Mol. Cell 39, 121–132 (2010).
Csordas, G., Thomas, A. P. & Hajnoczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 (1999).
Liu, Y. et al. DJ-1 regulates the integrity and function of ER-mitochondria association through interaction with IP3R3-Grp75-VDAC1. Proc. Natl Acad. Sci. USA 116, 25322–25328 (2019).
De Vos, K. J. et al. VAPB interacts with the mitochondrial protein PTPIP51 to regulate calcium homeostasis. Hum. Mol. Genet. 21, 1299–1311 (2012).
Jain, A. & Holthuis, J. C. M. Membrane contact sites, ancient and central hubs of cellular lipid logistics. Biochim. Biophys. Acta Mol. Cell Res. 1864, 1450–1458 (2017).
Stone, S. J. & Vance, J. E. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J. Biol. Chem. 275, 34534–34540 (2000).
Ardail, D., Lerme, F. & Louisot, P. Involvement of contact sites in phosphatidylserine import into liver mitochondria. J. Biol. Chem. 266, 7978–7981 (1991).
Steenbergen, R. et al. Disruption of the phosphatidylserine decarboxylase gene in mice causes embryonic lethality and mitochondrial defects. J. Biol. Chem. 280, 40032–40040 (2005).
Tasseva, G. et al. Phosphatidylethanolamine deficiency in mammalian mitochondria impairs oxidative phosphorylation and alters mitochondrial morphology. J. Biol. Chem. 288, 4158–4173 (2013).
Cui, Z., Vance, J. E., Chen, M. H., Voelker, D. R. & Vance, D. E. Cloning and expression of a novel phosphatidylethanolamine N-methyltransferase. a specific biochemical and cytological marker for a unique membrane fraction in rat liver. J. Biol. Chem. 268, 16655–16663 (1993).
Kornmann, B. et al. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481 (2009).
Jeong, H., Park, J., Jun, Y. & Lee, C. Crystal structures of Mmm1 and Mdm12–Mmm1 reveal mechanistic insight into phospholipid trafficking at ER-mitochondria contact sites. Proc. Natl Acad. Sci. USA 114, E9502–E9511 (2017).
Kawano, S. et al. Structure–function insights into direct lipid transfer between membranes by Mmm1–Mdm12 of ERMES. 217, 959–974 (2018).
Kopec, K. O., Alva, V. & Lupas, A. N. Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics 26, 1927–1931 (2010).
Jeong, H., Park, J., Jun, Y. & Lee, C. Crystal structures of Mmm1 and Mdm12-Mmm1 reveal mechanistic insight into phospholipid trafficking at ER-mitochondria contact sites. Proc. Natl Acad. Sci. USA 114, E9502–E9511 (2017).
Friedman, J. R. et al. ER tubules mark sites of mitochondrial division. Science 334, 358–362 (2011).
Abrisch, R. G., Gumbin, S. C., Wisniewski, B. T., Lackner, L. L. & Voeltz, G. K. Fission and fusion machineries converge at ER contact sites to regulate mitochondrial morphology. J. Cell Biol. 219, https://doi.org/10.1083/jcb.201911122 (2020).
Naon, D. et al. Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proc. Natl Acad. Sci. USA 113, 11249–11254 (2016).
Casellas-Diaz, S. et al. Mfn2 localization in the ER is necessary for its bioenergetic function and neuritic development. EMBO Rep. 22, e51954 (2021).
Lazarou, M., Jin, S. M., Kane, L. A. & Youle, R. J. Role of PINK1 binding to the TOM complex and alternate intracellular membranes in recruitment and activation of the E3 ligase Parkin. Dev. Cell 22, 320–333 (2012).
Chan, N. C. et al. Broad activation of the ubiquitin–proteasome system by Parkin is critical for mitophagy. 20, 1726-1737 (2011).
McLelland, G. L. et al. Mfn2 ubiquitination by PINK1/parkin gates the p97-dependent release of ER from mitochondria to drive mitophagy. Elife 7, https://doi.org/10.7554/eLife.32866 (2018).
Hamasaki, M. et al. Autophagosomes form at ER-mitochondria contact sites. Nature 495, 389–393 (2013).
Krebs, J., Agellon, L. B. & Michalak, M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochem. Biophys. Res. Commun. 460, 114–121 (2015).
Hayashi, T. & Su, T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131, 596–610 (2007).
Hirabayashi, Y. et al. ER-mitochondria tethering by PDZD8 regulates Ca(2+) dynamics in mammalian neurons. Science 358, 623–630 (2017).
Denton, R. M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta 1787, 1309–1316 (2009).
Cardenas, C. et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283 (2010).
Filadi, R. et al. TOM70 sustains cell bioenergetics by promoting IP3R3-mediated ER to mitochondria Ca(2+) Transfer. Curr. Biol. 28, 369–382.e366 (2018).
Baumgartner, H. K. et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J. Biol. Chem. 284, 20796–20803 (2009).
Giorgio, V. et al. Ca(2+) binding to F-ATP synthase beta subunit triggers the mitochondrial permeability transition. EMBO Rep. 18, 1065–1076 (2017).
Halestrap, A. P. What is the mitochondrial permeability transition pore? J. Mol. Cell. Cardiol. 46, 821–831 (2009).
Weinberg, F. & Chandel, N. S. Reactive oxygen species-dependent signaling regulates cancer. Cell. Mol. Life Sci. 66, 3663–3673 (2009).
Navarro-Yepes, J. et al. Antioxidant gene therapy against neuronal cell death. Pharmacol. Ther. 142, 206–230 (2014).
Pizzino, G. et al. Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 8416763 (2017).
Rajendran, P. et al. Antioxidants and human diseases. Clin. Chim. Acta 436, 332–347 (2014).
Taniyama, Y. & Griendling, K. K. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension 42, 1075–1081 (2003).
Valko, M., Rhodes, C. J., Moncol, J., Izakovic, M. & Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160, 1–40 (2006).
Bravo-Sagua, R. et al. Cell death and survival through the endoplasmic reticulum-mitochondrial axis. Curr. Mol. Med. 13, 317–329 (2013).
Verfaillie, T. et al. PERK is required at the ER-mitochondrial contact sites to convey apoptosis after ROS-based ER stress. Cell Death Differ. 19, 1880–1891 (2012).
Pagani, M. et al. Endoplasmic reticulum oxidoreductin 1-lbeta (ERO1-Lbeta), a human gene induced in the course of the unfolded protein response. J. Biol. Chem. 275, 23685–23692 (2000).
Molinari, M. & Helenius, A. Glycoproteins form mixed disulphides with oxidoreductases during folding in living cells. Nature 402, 90–93 (1999).
Zito, E. ERO1: a protein disulfide oxidase and H2O2 producer. Free Radic. Biol. Med. 83, 299–304 (2015).
Li, G. et al. Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J. Cell Biol. 186, 783–792 (2009).
Higo, T. et al. Subtype-specific and ER lumenal environment-dependent regulation of inositol 1,4,5-trisphosphate receptor type 1 by ERp44. Cell 120, 85–98 (2005).
J, O. U., Ryu, S. Y., Jhun, B. S., Hurst, S. & Sheu, S. S. Mitochondrial ion channels/transporters as sensors and regulators of cellular redox signaling. Antioxid. Redox Signal. 21, 987–1006 (2014).
Joseph, S. K., Booth, D. M., Young, M. P. & Hajnoczky, G. Redox regulation of ER and mitochondrial Ca(2+) signaling in cell survival and death. Cell Calcium 79, 89–97 (2019).
Meunier, J. & Hayashi, T. Sigma-1 receptors regulate Bcl-2 expression by reactive oxygen species-dependent transcriptional regulation of nuclear factor kappaB. J. Pharmacol. Exp. Ther. 332, 388–397 (2010).
Ke, M., He, G., Wang, H., Cheng, S. & Xu, Y. Sigma receptor knockdown augments dysfunction and apoptosis of beta cells induced by palmitate. Exp. Biol. Med. 246, 1491–1499 (2021).
Goguadze, N., Zhuravliova, E., Morin, D., Mikeladze, D. & Maurice, T. Sigma-1 receptor agonists induce oxidative stress in mitochondria and enhance complex I activity in physiological condition but protect against pathological oxidative stress. Neurotox. Res. 35, 1–18 (2019).
Nemoto, S. et al. The mammalian longevity-associated gene product p66shc regulates mitochondrial metabolism. J. Biol. Chem. 281, 10555–10560 (2006).
Khalid, S. et al. cJun N-terminal kinase (JNK) phosphorylation of serine 36 is critical for p66Shc activation. Sci. Rep. 6, 20930 (2016).
Khanday, F. A. et al. Rac1 leads to phosphorylation-dependent increase in stability of the p66shc adaptor protein: role in Rac1-induced oxidative stress. Mol. Biol. Cell 17, 122–129 (2006).
Natalicchio, A., Tortosa, F., Perrini, S., Laviola, L. & Giorgino, F. p66Shc, a multifaceted protein linking Erk signalling, glucose metabolism, and oxidative stress. Arch. Physiol. Biochem. 117, 116–124 (2011).
Giorgio, M. et al. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell 122, 221–233 (2005).
Francia, P. et al. Deletion of p66shc gene protects against age-related endothelial dysfunction. Circulation 110, 2889–2895 (2004).
Napoli, C. et al. Deletion of the p66Shc longevity gene reduces systemic and tissue oxidative stress, vascular cell apoptosis, and early atherogenesis in mice fed a high-fat diet. Proc. Natl Acad. Sci. USA 100, 2112–2116 (2003).
Ishihara, N., Eura, Y. & Mihara, K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J. Cell Sci. 117, 6535–6546 (2004).
Munoz, J. P. et al. Mfn2 modulates the UPR and mitochondrial function via repression of PERK. EMBO J. 32, 2348–2361 (2013).
Xin, Y. et al. Inhibition of mitofusin-2 promotes cardiac fibroblast activation via the PERK/ATF4 pathway and reactive oxygen species. Oxidative Med. Cell. Longev. 2019, 3649808 (2019).
Tur, J. et al. Mitofusin 2 in macrophages links mitochondrial ROS production, cytokine release, phagocytosis, autophagy, and bactericidal activity. Cell Rep. 32, 108079 (2020).
Paschen, W. & Mengesdorf, T. Endoplasmic reticulum stress response and neurodegeneration. Cell Calcium 38, 409–415 (2005).
So, J. S. Roles of endoplasmic reticulum stress in immune responses. Mol. Cells 41, 705–716 (2018).
Xia, S., Duan, W., Liu, W., Zhang, X. & Wang, Q. GRP78 in lung cancer. J. Transl. Med. 19, 118 (2021).
Shen, J., Chen, X., Hendershot, L. & Prywes, R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell 3, 99–111 (2002).
Hetz, C. & Glimcher, L. H. Fine-tuning of the unfolded protein response: assembling the IRE1alpha interactome. Mol, Cell 35, 551–561 (2009).
Rozpedek, W. et al. The role of the PERK/eIF2alpha/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 16, 533–544 (2016).
Liu, Z. W. et al. Protein kinase RNA-like endoplasmic reticulum kinase (PERK) signaling pathway plays a major role in reactive oxygen species (ROS)-mediated endoplasmic reticulum stress-induced apoptosis in diabetic cardiomyopathy. Cardiovasc. Diabetol. 12, 158 (2013).
Mitsuda, T. et al. Sigma-1Rs are upregulated via PERK/eIF2alpha/ATF4 pathway and execute protective function in ER stress. Biochem. Biophys. Res. Commun. 415, 519–525 (2011).
Chen, P. et al. Rab32 promotes glioblastoma migration and invasion via regulation of ERK/Drp1-mediated mitochondrial fission. Cell Death Dis. 14, 198 (2023).
Haile, Y. et al. Rab32 connects ER stress to mitochondrial defects in multiple sclerosis. J. Neuroinflammation 14, 19 (2017).
Higo, T. et al. Mechanism of ER stress-induced brain damage by IP(3) receptor. Neuron 68, 865–878 (2010).
Kanekura, K., Nishimoto, I., Aiso, S. & Matsuoka, M. Characterization of amyotrophic lateral sclerosis-linked P56S mutation of vesicle-associated membrane protein-associated protein B (VAPB/ALS8). J. Biol. Chem. 281, 30223–30233 (2006).
Gkogkas, C. et al. VAPB interacts with and modulates the activity of ATF6. Hum. Mol. Genet. 17, 1517–1526 (2008).
Jones, R. G. & Thompson, C. B. Tumor suppressors and cell metabolism: a recipe for cancer growth. Gene Dev. 23, 537–548 (2009).
Fu, Y. et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm (2020) 2, 27–59 (2021).
Bong, A. H. L. & Monteith, G. R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. Cell. Res. 1865, 1786–1794 (2018).
Monteith, G. R., Prevarskaya, N. & Roberts-Thomson, S. J. The calcium-cancer signalling nexus. Nat. Rev. Cancer 17, 367–380 (2017).
Simoes, I. C. M. et al. The mystery of mitochondria-ER contact sites in physiology and pathology: a cancer perspective. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165834 (2020).
Kuchay, S. et al. PTEN counteracts FBXL2 to promote IP3R3-and Ca2+-mediated apoptosis limiting tumour growth. Nature 546, 554 (2017).
Salmena, L., Carracedo, A. & Pandolfi, P. P. Tenets of PTEN tumor suppression. Cell 133, 403–414 (2008).
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E. & Rizzuto, R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene 27, 6407–6418 (2008).
Arbel, N. & Shoshan-Barmatz, V. Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J. Biol. Chem. 285, 6053–6062 (2010).
Bononi, A. et al. BAP1 regulates IP3R3-mediated Ca(2+) flux to mitochondria suppressing cell transformation. Nature 546, 549–553 (2017).
Kobrinski, D. A., Yang, H. & Kittaneh, M. BAP1: role in carcinogenesis and clinical implications. Transl. Lung Cancer Res. 9, S60–S66 (2020).
D’Eletto, M. et al. Transglutaminase type 2 regulates ER-mitochondria contact sites by interacting with GRP75. Cell Rep. 25, 3573–3581.e3574 (2018).
Mangala, L. S., Fok, J. Y., Zorrilla-Calancha, I. R., Verma, A. & Mehta, K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 26, 2459–2470 (2007).
Sotgia, F. et al. Mitochondrial metabolism in cancer metastasis visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle 11, 1445–1454 (2012).
Liu, Y. et al. MCU-induced mitochondrial calcium uptake promotes mitochondrial biogenesis and colorectal cancer growth. Signal Transduct. Target. Ther. 5, 59 (2020).
Stewart, T. A., Yapa, K. T. & Monteith, G. R. Altered calcium signaling in cancer cells. Biochim. Biophys. Acta 1848, 2502–2511 (2015).
Chiu, H. Y., Loh, A. H. P. & Taneja, R. Mitochondrial calcium uptake regulates tumour progression in embryonal rhabdomyosarcoma. Cell Death Dis. 13, https://doi.org/10.1038/s41419-022-04835-4 (2022).
Doghman-Bouguerra, M. & Lalli, E. ER-mitochondria interactions: both strength and weakness within cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 1866, 650–662 (2019).
Hojo, Y. et al. Sunitinib and pterostilbene combination treatment exerts antitumor effects in gastric cancer via suppression of PDZD8. Int. J. Mol. Sci. 23, https://doi.org/10.3390/ijms23074002 (2022).
Nemoto, S. & Finkel, T. Redox regulation of forkhead proteins through a p66shc-dependent signaling pathway. Science 295, 2450–2452 (2002).
Veeramani, S., Yuan, T. C., Lin, F. F. & Lin, M. F. Mitochondrial redox signaling by p66Shc is involved in regulating androgenic growth stimulation of human prostate cancer cells. Oncogene 27, 5057–5068 (2008).
Miller, D. R. et al. p66Shc protein through a redox mechanism enhances the progression of prostate cancer cells towards castration-resistance. Free Radical. Biol. Med. 139, 24–34 (2019).
Ingersoll, M. A. et al. p66Shc regulates migration of castration-resistant prostate cancer cells. Cell. Signal. 46, 1–14 (2018).
Davol, P. A. Shc proteins are strong, independent prognostic markers for both node-negative and node-positive primary breast cancer (vol 63, pg 6772, 2003). Cancer Res. 63, 8562–8562 (2003).
Haines, E., Saucier, C. & Claing, A. The adaptor proteins p66Shc and Grb2 regulate the activation of the GTPases ARF1 and ARF6 in invasive breast cancer cells. J. Biol. Chem. 289, 5687–5703 (2014).
Zheng, Z. et al. Downregulated adaptor protein p66(Shc) mitigates autophagy process by low nutrient and enhances apoptotic resistance in human lung adenocarcinoma A549 cells. FEBS J. 280, 4522–4530 (2013).
Yan, W. D. et al. Expression of endoplasmic reticulum oxidoreductase 1-alpha in cholangiocarcinoma tissues and its effects on the proliferation and migration of cholangiocarcinoma cells. Cancer Manag. Res. 11, 6727–6739 (2019).
Varone, E. et al. The ER stress response mediator ERO1 triggers cancer metastasis by favoring the angiogenic switch in hypoxic conditions. Oncogene 40, 1721–1736 (2021).
Yang, S. K. et al. Endoplasmic reticulum resident oxidase ERO1-Lalpha promotes hepatocellular carcinoma metastasis and angiogenesis through the S1PR1/STAT3/VEGF-A pathway. Cell Death Dis. 9, https://doi.org/10.1038/s41419-018-1134-4 (2018).
Johnson, B. D., Geldenhuys, W. J. & Hazlehurst, L. A. The Role of ERO1alpha in modulating cancer progression and immune escape. J. Cancer Immunol. 2, 103–115 (2020).
Snaebjornsson, M. T., Janaki-Raman, S. & Schulze, A. Greasing the wheels of the cancer machine: the role of lipid metabolism in cancer. Cell Metab. 31, 62–76 (2020).
Bauerschlag, D. O. et al. Fatty acid synthase overexpression: target for therapy and reversal of chemoresistance in ovarian cancer. J. Transl. Med. 13, 146 (2015).
Rohrig, F. & Schulze, A. The multifaceted roles of fatty acid synthesis in cancer. Nat. Rev. Cancer 16, 732–749 (2016).
Rusinol, A. E., Cui, Z., Chen, M. H. & Vance, J. E. A unique mitochondria-associated membrane-fraction from rat-liver has a high-capacity for lipid-synthesis and contains pre-golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 269, 27494–27502 (1994).
Lewin, T. M., Kim, J. H., Granger, D. A., Vance, J. E. & Coleman, R. A. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276, 24674–24679 (2001).
Saddoughi, S. A. & Ogretmen, B. Diverse functions of ceramide in cancer cell death and proliferation. Adv. Cancer Res. 117, 37–58 (2013).
Haimovitz-Friedman, A., Kolesnick, R. N. & Fuks, Z. Ceramide signaling in apoptosis. Br. Med. Bull. 53, 539–553 (1997).
Puglielli, L. et al. Acyl-coenzyme A : cholesterol acyltransferase modulates the generation of the amyloid beta-peptide. Nat. Cell Biol. 3, 905–912 (2001).
Zabielska, J., Sledzinski, T. & Stelmanska, E. Acyl-Coenzyme A: cholesterol acyltransferase inhibition in cancer treatment. Anticancer Res. 39, 3385–3394 (2019).
Nwosu, Z. C., Ebert, M. P., Dooley, S. & Meyer, C. Caveolin-1 in the regulation of cell metabolism: a cancer perspective. Mol. Cancer 15, 71 (2016).
Patani, N., Martin, L. A., Reis-Filho, J. S. & Dowsett, M. The role of caveolin-1 in human breast cancer. Breast Cancer Res. Treat. 131, 1–15 (2012).
Wang, Z. et al. Caveolin-1, a stress-related oncotarget, in drug resistance. Oncotarget 6, 37135–37150 (2015).
Tang, Y. et al. Caveolin-1 is related to invasion, survival, and poor prognosis in hepatocellular cancer. Med. Oncol. 29, 977–984 (2012).
Yadav, R. K., Chae, S. W., Kim, H. R. & Chae, H. J. Endoplasmic reticulum stress and cancer. J. Cancer Prev. 19, 75–88 (2014).
Crottes, D. et al. SIGMAR1 regulates membrane electrical activity in response to extracellular matrix stimulation to drive cancer cell invasiveness. Cancer Res. 76, 607–618 (2016).
Rao, M. et al. VAMP-associated protein B (VAPB) promotes breast tumor growth by modulation of Akt activity. PLoS One 7, e46281 (2012).
Dasari, S. & Tchounwou, P. B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378 (2014).
Xu, L. et al. Bcl-2 overexpression reduces cisplatin cytotoxicity by decreasing ER-mitochondrial Ca2+ signaling in SKOV3 cells. Oncol. Rep. 39, 985–992 (2018).
Giorgi, C. et al. p53 at the endoplasmic reticulum regulates apoptosis in a Ca2+-dependent manner. Proc. Natl Acad. Sci. USA 112, 1779–1784 (2015).
Marei, H. E. et al. p53 signaling in cancer progression and therapy. Cancer Cell Int. 21, 703 (2021).
Doan, N. T. et al. Targeting thapsigargin towards tumors. Steroids 97, 2–7 (2015).
Fang, Y. T. et al. Overexpressed VDAC1 in breast cancer as a novel prognostic biomarker and correlates with immune infiltrates. World J. Surg. Oncol. 20, https://doi.org/10.1186/s12957-022-02667-2 (2022).
Shoshan-Barmatz, V., Krelin, Y., Shteinfer-Kuzmine, A. & Arif, T. Voltage-dependent anion channel 1 as an emerging drug target for novel anti-cancer therapeutics. Front. Oncol. 7, 154 (2017).
Wu, H., Chen, W., Chen, Z., Li, X. & Wang, M. Novel tumor therapy strategies targeting endoplasmic reticulum-mitochondria signal pathways. Ageing Res. Rev. 88, 101951 (2023).
Sbiera, S. et al. Mitotane inhibits Sterol-O-Acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology 156, 3895–3908 (2015).
Doghman-Bouguerra, M. et al. FATE1 antagonizes calcium- and drug-induced apoptosis by uncoupling ER and mitochondria. EMBO Rep. 17, 1264–1280 (2016).
Ma, B. et al. Corosolic acid, a natural triterpenoid, induces ER stress-dependent apoptosis in human castration resistant prostate cancer cells via activation of IRE-1/JNK, PERK/CHOP and TRIB3. J. Exp. Clin. Cancer Res. 37, 210 (2018).
Zhu, G. Y. et al. 20(S)-Protopanaxadiol, a metabolite of ginsenosides, induced cell apoptosis through endoplasmic reticulum stress in human hepatocarcinoma HepG2 cells. Eur. J. Pharmacol. 668, 88–98 (2011).
Kim, H. S. et al. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int. J. Cancer 138, 1432–1441 (2016).
Martucciello, S., Masullo, M., Cerulli, A. & Piacente, S. Natural Products Targeting ER Stress, and the Functional Link to Mitochondria. Int. J. Mol. Sci. 21, https://doi.org/10.3390/ijms21061905 (2020).
Kim, H. et al. Evodiamine Eliminates Colon Cancer Stem Cells via Suppressing Notch and Wnt Signaling. Molecules 24, https://doi.org/10.3390/molecules24244520 (2019).
Yang, X. et al. Mitochondria-associated endoplasmic reticulum membrane: overview and inextricable link with cancer. J. Cell Mol. Med. 27, 906–919 (2023).
Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 68, 284–296 (2018).
Yang, L., Li, H., Jiang, Y., Zuo, J. & Liu, W. Inhibition of mortalin expression reverses cisplatin resistance and attenuates growth of ovarian cancer cells. Cancer Lett. 336, 213–221 (2013).
Li, J. et al. GRP75-faciliated mitochondria-associated ER membrane (MAM) integrity controls cisplatin-resistance in ovarian cancer patients. Int. J. Biol. Sci. 18, 2914–2931 (2022).
Gil-Hernandez, A. et al. Relevance of Membrane Contact Sites in Cancer Progression. Front. Cell Dev. Biol. 8, https://doi.org/10.3389/fcell.2020.622215 (2021).
Xie, Q. et al. ABT737 reverses cisplatin resistance by regulating ER-mitochondria Ca2+ signal transduction in human ovarian cancer cells. Int. J. Oncol. 49, 2507–2519 (2016).
Fan, P. & Jordan, V. C. PERK, beyond an unfolded protein response sensor in estrogen-induced apoptosis in endocrine-resistant breast cancer. Mol. Cancer Res. 20, 193–201 (2022).
Fan, P. et al. Modulation of nuclear factor-kappa B activation by the endoplasmic reticulum stress sensor PERK to mediate estrogen-induced apoptosis in breast cancer cells. Cell Death Discov. 4, 15 (2018).
Fan, P. & Jordan, V. C. How PERK kinase conveys stress signals to nuclear factor-kappa B to mediate estrogen-induced apoptosis in breast cancer cells? Cell Death Dis. 9, https://doi.org/10.1038/s41419-018-0516-y (2018).
Nawrocki, S. T. et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 65, 11658–11666 (2005).
Paupe, V. & Prudent, J. New insights into the role of mitochondrial calcium homeostasis in cell migration. Biochem. Biophys. Res. Commun. 500, 75–86 (2018).
Tosatto, A. et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1. Embo. Mol. Med. 8, 569–585 (2016).
Wu, L. et al. FUN14 domain-containing 1 promotes breast cancer proliferation and migration by activating calcium-NFATC1-BMI1 axis. EBioMedicine 41, 384–394 (2019).
Wu, W. X. et al. FUNDC1 is a novel mitochondrial-associated-membrane (MAM) protein required for hypoxia-induced mitochondrial fission and mitophagy. Autophagy 12, 1675–1676 (2016).
Bassoy, E. Y. et al. ER-mitochondria contacts control surface glycan expression and sensitivity to killer lymphocytes in glioma stem-like cells. EMBO J. 36, 1493–1512 (2017).
Bantug, G. R. et al. Mitochondria-endoplasmic reticulum contact sites function as immunometabolic hubs that orchestrate the rapid recall response of memory CD8(+) T cells. Immunity 48, 542 (2018).
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grants funded by the Korean government (MSIT) (grant numbers: 2021R1A2C2005841 and 2021R1C1C1009807). Additionally, this research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI22C1424).
Author information
Authors and Affiliations
Contributions
G.A., J.P. J.S., and T.H. wrote the manuscript and prepared the figures and tables. G.A. and J.P. edited the manuscript and figures. G.S. and W.L. conceived, structured and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
An, G., Park, J., Song, J. et al. Relevance of the endoplasmic reticulum-mitochondria axis in cancer diagnosis and therapy. Exp Mol Med 56, 40–50 (2024). https://doi.org/10.1038/s12276-023-01137-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-01137-3