Abstract
Proteases are involved in almost all biological processes, implying their importance for both health and pathological conditions. Dysregulation of proteases is a key event in cancer. Initially, research identified their role in invasion and metastasis, but more recent studies have shown that proteases are involved in all stages of cancer development and progression, both directly through proteolytic activity and indirectly via regulation of cellular signaling and functions. Over the past two decades, a novel subfamily of serine proteases called type II transmembrane serine proteases (TTSPs) has been identified. Many TTSPs are overexpressed by a variety of tumors and are potential novel markers of tumor development and progression; these TTSPs are possible molecular targets for anticancer therapeutics. The transmembrane protease serine 4 (TMPRSS4), a member of the TTSP family, is upregulated in pancreatic, colorectal, gastric, lung, thyroid, prostate, and several other cancers; indeed, elevated expression of TMPRSS4 often correlates with poor prognosis. Based on its broad expression profile in cancer, TMPRSS4 has been the focus of attention in anticancer research. This review summarizes up-to-date information regarding the expression, regulation, and clinical relevance of TMPRSS4, as well as its role in pathological contexts, particularly in cancer. It also provides a general overview of epithelial-mesenchymal transition and TTSPs.
Similar content being viewed by others
Epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) is a reversible cellular process during which organized epithelial cells lose apical-basal polarity, decrease cell-cell adhesion, and reorganize their cytoskeleton while simultaneously individually or collectively acquiring mesenchymal features such as fibroblast-like morphology, increased cell motility, and invasive capability1,2,3. Cells undergoing EMT show reduced expression or function of epithelial markers such as E-cadherin, ZO-1, and certain cytokeratins, along with increased expression of mesenchymal markers such as vimentin, fibronectin, N-cadherin, and certain integrins.
EMT plays critical roles during developmental processes, including gastrulation, somitogenesis, and neural crest migration, as well as during epithelial wound healing in adult organisms1. Furthermore, EMT is activated during pathological processes such as cancer and organ fibrosis.
In cancer cells, EMT is rarely activated through a cell-autonomous process. Rather, EMT is instigated by paracrine signaling factors derived from the tumor-associated reactive stroma; notably, EMT events are induced by transforming growth factor-β (TGF-β) and other factors, including WNT proteins, hypoxia, growth factors, cytokines, and extracellular matrix (ECM)-integrin adhesions4,5. Consequently, these factors induce the expression and functional activation of EMT-inducing transcription factors (EMT-TFs), which can pleiotropically orchestrate diverse transcriptional changes associated with EMT. The major group of EMT-TFs includes members of the zinc-finger-binding transcription factors (i.e., the SNAIL family: SNAIL encoded by SNAI1 and SLUG encoded by SNAI2), the TWIST family of basic helix-loop-helix factors (TWIST1 and TWIST2), and the zinc-finger E-box-binding homeobox factors (the ZEB family: ZEB1 and ZEB2). Other transcription factors, such as Forkhead box C (FOXC2) and paired-related homeobox 1 (PRRX1), can also induce EMT6. These factors display distinct expression profiles and target gene patterns depending on the cell and tissue type.
In addition, proteases are involved in EMT induction5,7; for example, matrix metalloproteinase (MMP)−3 triggers EMT by increasing the cellular levels of reactive oxygen species (ROS), leading to upregulation of SNAIL. MMP-14 and MMP-8 induce EMT through activation of TGF-β signaling. IL-6-induction of a disintegrin and metalloprotease (ADAM) 9 expression leads to ROS production and EMT. ADAM10 cleaves the ectodomain of E-cadherin, which modulates cell-cell adhesion, cell migration, β-catenin translocation/signaling, and EMT7. The membrane-bound serine protease TMPRSS4 induces EMT in colon and hepatocellular carcinoma cells, which is accompanied by upregulation of ZEB2, SNAIL, and SLUG8,9. TMPRSS3 induces EMT in gastric cancer cells through regulation of the ERK1/2 and AKT pathways10. TMPRSS2 induces proinvasive EMT and metastasis in a prostate cancer model, potentially through prohepatocyte growth factor (HGF) activation and subsequent c-MET signaling11.
EMT-TFs are additionally regulated by miRNAs (miRs) and long noncoding RNAs (lncRNAs), epigenetic modifications, alternative splicing, and posttranslational regulation of protein stability. For example, miR-200s and ZEB1/2 form a double-negative feedback loop in which ZEB1/2 inhibits the transcription of miR-200 mRNAs, and miR-200s suppress ZEB1 expression. Similarly, a double-negative feedback loop is established between miR-1 and miR-200 and SNAI2. Such mechanisms allow rapid and robust enforcement of epithelial and mesenchymal states in response to minimal signals1. In addition, ZEB1 can switch from a transcriptional repressor to an activator by interacting with YAP112 or the coactivators PCAF and p3001. ZEB2 cooperates with SP1 to function as a transcriptional activator to promote expression of the mesenchymal markers integrin α5 and vimentin13,14.
The roles of EMT or EMT-TFs in cancer progression are not limited to the regulation of cancer cell migration/invasion and dissemination; instead, these factors play multiple pivotal roles, including regulating tumor initiation, cancer stem cell (CSC) plasticity, cell fate specification, therapy resistance, and immune evasion3,6, although the underlying molecular mechanisms remain unclear. Of particular interest is the relationship between EMT, CSC status, and malignant progression. Certain types of cancer cells, such as breast, colorectal, ovarian, pancreatic, and prostate cancer cells, can acquire tumor-initiating capability after induction of EMT programs6. Overexpression of SNAI1, SNAI2, or TWIST in immortalized or transformed human mammary epithelial cells leads to a stem cell phenotype, which facilitates sphere formation and induces the expression of stem cell markers1. ZEB1 also confers stem cell-like properties and tumorigenicity on mouse and human pancreatic and colorectal cancer cells by suppressing stemness-inhibiting miRNAs such as miR-203, miR-200s, and miR-183, which repress the expression of stemness factors such as BMI1, SOX2, and KLF415. TWIST1 upregulates BMI1, which is essential for promoting EMT and tumor-initiating capability in head and neck squamous cell carcinoma16 and hepatocellular carcinoma17 cells. Elevated SLUG expression correlates with the overexpression of stem-like genes, including CD133 and BMI1, in basal-type breast tumors18. SLUG is required for SOX9 stabilization, which supports CSCs and promotes metastasis in lung cancer19.
Malignant progression of most types of carcinoma depends on tumor cell EMT. In the context of metastasis, while EMT is involved in invasion, dissemination, and extravasation, the reverse process (mesenchymal-epithelial transition (MET)) is induced by either cell-intrinsic or stromal components; this process is thought to be required for metastatic colonization by disseminated cancer cells1,3. In this regard, it has been suggested6,20 that due to the multilayered regulatory network, EMT is a dynamic program that is (usually) partially activated and likely to generate cells residing in an intermediate, partially epithelial and partially mesenchymal phenotypic state (referred to as epithelial-mesenchymal plasticity (EMP)). This partial EMT phenotypic state is highly variable, changing based on the context, even within individual tumors. Partial/incomplete EMT or EMP is widely observed during development, wound healing, and cancer6,21. Importantly, cancer cells that undergo partial EMT are thought to have higher metastatic potential than fully mesenchymal cells, partially due to their ability to migrate in clusters and enhanced attachment to the ECM. Induction of the partial EMT phenotype is also associated with CSC expansion and subsequent metastatic colonization, as well as resistance to chemotherapy and targeted therapies6,20.
The type II transmembrane serine protease (TTSP) family
Proteases are involved in almost all biological processes. The human genome contains approximately 700 peptidases (known and putative) in the MEROPS database22; among these, 178 are serine proteases, most of which (138) belong to the S1 family of trypsin-like proteases23. Serine proteases play critical roles in digestion, blood coagulation, fibrinolysis, development, immunity, tissue regeneration, and inflammation23. Dysregulation of protease activity causes cardiovascular and inflammatory diseases, cancer, osteoporosis, and neurological disorders24,25.
Most members of the serine protease family are either secreted or compartmentalized in cytoplasmic organelles. A subgroup of serine proteases is anchored directly to the plasma membrane. These cell surface-anchored serine proteases are tethered via a C-terminal transmembrane domain (type I), an amino-terminal transmembrane domain (type II), or a C-terminal hydrophobic region that functions as a signal for membrane attachment via a glycosyl-phosphatidylinositol linkage (GPI-anchored)26.
TTSPs, the largest group of membrane-anchored serine proteases, share a common structural architecture comprising a short intracellular N-terminal domain followed by a single-pass hydrophobic transmembrane domain, a variable-length stem region containing modular structural domains, and a C-terminal extracellular proteolytic domain that is displayed on the cell surface. The catalytic domain of TTSPs belongs to the S1 family of serine proteases26. Enteropeptidase (also known as enterokinase), which was identified over a century ago due to its critical role in food digestion, was the first identified TTSP (it was identified three decades ago by molecular cloning of enteropeptidase cDNA). TMPRSS2, TMPRSS11D/HAT, corin, and matriptase were subsequently identified as cell surface-anchored proteases27.
Humans express 17 TTSPs, which are divided into four different subfamilies: the (i) matriptase, (ii) hepsin/transmembrane protease, serine (TMPRSS), (iii) human airway trypsin-like (HAT)/differentially expressed in squamous cell carcinoma (DESC), and (iv) corin subfamilies28. The proteolytic domain is highly conserved between different TTSPs, and their proteolytic activity is dependent on a catalytic triad comprising His, Asp, and Ser. TTSPs possess a conserved Asp residue at the bottom of the S1 substrate-binding pocket, resulting in a cleavage preference for substrates that have Arg or Lys in the P1 position. TTSPs are expressed as single-chain, inactive zymogens that are activated proteolytically by cleavage of a positively charged residue (Arg or Lys) located in a conserved activation motif between the prodomain and catalytic domain; cleavage releases the active mature protein. After activation, they are predicted to remain bound to the cell membrane through conserved disulfide bonds that link the catalytic domain to the rest of the extracellular domain. Several TTSPs, including matriptase, hepsin/TMPRSS1, TMPRSS2, TMPRSS3, TMPRSS4, matriptase-2/TMPRSS6, and TMPRSS13, are capable of autoactivation26,29, indicating that their respective zymogens have baseline activity. On the other hand, the soluble forms of several TTSP members have been detected in vivo, implying that the extracellular domains may also be shed from the cell surface30. For example, in prostate cancer, soluble TMPRSS2 is generated by autocatalytic cleavage and released into the circulation31. In addition, TTSPs are regulated by endogenous protease inhibitors such as Kunitz domain-containing inhibitors and the serpin family. The membrane-associated Kunitz-type inhibitors hepatocyte growth factor activator inhibitor type I (HAI-1) and HAI-2 are thought to inhibit matriptase and hepsin32.
Expression of TTSPs is widespread throughout the human body and is enriched in epithelial tissues of various origins. Several members of the TTSP family play critical biological roles in diverse processes, including epithelial differentiation and barrier function, tissue homeostasis, iron metabolism, hearing, and blood pressure regulation33. Their dysregulated expression has been associated with diverse disorders, including cancer, skin defects, anemia, deafness, hypertension, and obesity34.
TTSPs in cancer: matriptase
Several TTSPs are associated with cancer progression; indeed, early reports show that several members of the TTSP family are highly and selectively expressed by a variety of tumors relative to the corresponding normal tissues, indicating their potential as novel markers of tumor development and progression. In addition to expression analysis and basic biochemical characterization, studies have examined the functions of TTSPs in physiological and disease-related conditions, focusing on the identification of proteolytic substrates, deregulation of proteases, characterization of cognate inhibitors, and dissection of underlying intracellular signaling pathways. More recently, studies of genetically modified mice and mice treated with protease inhibitors have demonstrated that the dysregulated expression of TTSPs is causally associated with cancer initiation and progression26,28,33,35.
Matriptase, also known as MT-SP1, CAP3, TADG15, or epithin (gene name: ST14), is the most studied member of the TTSP family. Matriptase is expressed in the epithelial compartment in a wide variety of tissues and is upregulated in breast, prostate, ovarian, uterine, colon, cervical, gastric, head and neck, pancreatic, and skin cancers. Increased expression of matriptase is associated with a poor prognosis. In several cancers, the ratio of matriptase to its endogenous inhibitors HAI-1 and HAI-2 is increased, whereas the ratio is low in normal tissues; this suggests that an imbalance between matriptase and its endogenous inhibitors contributes to tumorigenesis28,33. Mice with transgenic expression of matriptase in the epidermis develop spontaneous squamous cell carcinoma and display susceptibility to carcinogen-induced tumorigenesis. In addition, matriptase-mediated tumorigenesis is completely blocked or impaired in double transgenic mice that concomitantly express transgenic epidermal HAI-1 or HAI-2, demonstrating the tumor-promoting role of matriptase/inhibitor imbalance36.
A study based on a mouse model of invasive ductal mammary carcinoma (MMTV-PymT) showed that matriptase hypomorphic mice with low levels of matriptase display a significant delay in tumor formation and blunted tumor growth due to reduced cancer cell proliferation37. Matriptase critically contributes to breast cancer progression via activation of the HGF/c-MET signaling pathway; upon proteolytic cleavage by matriptase, pro-HGF is converted to active HGF, which initiates c-MET signaling and activates downstream targets such as PI3K/AKT and Gab1, thereby contributing to cancer progression37. Among TTSPs, matriptase displays the most efficient pro-HGF processing activity32; it also activates pro-forms of urokinase plasminogen activator (uPA), macrophage stimulating protein 1 (MSP-1), protease-activated receptor 2 (PAR-2), and prostasin. Activation of uPA, MSP-1, and PAR-2 initiates downstream signaling pathways, such as plasmin activation, the MSP-1/RON pathway, and the PAR-2/NF-κB inflammatory pathway, respectively. In addition, other cancer-related proteins, including SRC-associated protein CUB domain-containing protein 1 (CDCP1/SIMA135/TRASK), platelet-derived growth factor (PDGF)-C and PDGF-D, vascular endothelial growth factor receptor 2 (VEGFR2), and insulin-like growth factor-binding protein-related protein-1 (IGFBP-rP1), are potential substrates of matriptase38. Active matriptase degrades the ECM components fibronectin and laminin in vitro28, suggesting a role in direct remodeling of the ECM and cancer cell invasion.
Matriptase is expressed widely in various organs and is critical for organ development. Matriptase-deficient mice are perinatal lethal and exhibit abnormal terminal differentiation of epidermal keratinocytes, increased thymocyte apoptosis, and loss of epidermal barrier function, leading to fatal postnatal dehydration39. These effects should be considered carefully if matriptase blockade is to be developed as an anticancer therapy.
Role of TTSPs in viral infections
TTSPs expressed in the human respiratory tract are implicated in viral spread due to their ability to cleave the surface proteins of the virus, which mediates virus entry into cells via direct fusion at the plasma membrane surface. Thus, some TTSPs expressed in the human airway facilitate the entry and spread of influenza viruses and coronaviruses, including SARS-CoV-2. TMPRSS2, matriptase, TMPRSS11A, DESC1, TMPRSS11D/HAT, and TMPRSS13 cleave the spike protein of SARS-CoVs and/or MERS-CoVs in vitro, allowing the viruses to enter and replicate in airway epithelial cells34,40. TMPRSS2 is a potential target for the treatment of COVID-19 due to its involvement in viral spike protein processing and localization in human epithelial tissues of the respiratory, genitourinary, and gastrointestinal tracts and its coexpression with the coronavirus receptor hACE2 in lung tissues. TMPRSS2 mediates viral entry through two distinct mechanisms: proteolytic cleavage of ACE2, which enhances viral uptake, and cleavage of the spike protein, which triggers host cell entry via membrane fusion41. Mice lacking TMPRSS2 exhibit no abnormalities in the absence of infection42, suggesting that blocking TMPRSS2 may not have significant undesired side effects. Thus, inhibitors of TMPRSS2 may be a potential treatment for COVID-19. TTSPs with similar cleavage specificity are coexpressed in many tissues, including the human airway34, implying functional redundancy at certain levels. Therefore, TMPRSS2 may not be the sole protease that controls spike priming; hence, blocking TMPRSS2 alone may not be effective.
Similar to the coronavirus spike protein, the surface protein of influenza A and B viruses (hemagglutinin (HA)) also requires cleavage by host proteases to mediate entry into respiratory epithelial cells. Among TTSPs, TMPRSS2, TMPRSS4, matriptase, TMPRSS11A, TMPRSS11D/HAT, DESC1, and TMPRSS13 can cleave HA in vitro or in vivo34,40,43.
Transmembrane protease serine 4 (TMPRSS4)
Expression and regulation of TMPRSS4 in cancer
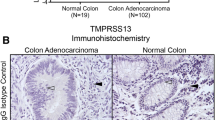
TMPRSS4 (gene ID 56649; chromosomal location 11q23.3), initially referred to as TMPRSS3 but also known as CAP2 or MT-SP2, was originally identified as a gene strongly expressed by most pancreatic carcinoma tissues but not in the normal pancreas or in cases of chronic pancreatitis44. Among the seven isoforms reported thus far, isoform 1 represents the longest transcript, encoding a 437 amino acid protein that contains an extracellular region harboring a trypsin-like serine protease domain. In humans, TMPRSS4 mRNA has been detected in the esophagus, stomach, small intestine, colon, bladder, and kidney44, although the physiological roles of TMPRSS4 remain unclear. Based on an analysis of The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) datasets, Katopodis et al.45 reported that TMPRSS4 is upregulated significantly in 11 types of cancer, including lung adenocarcinoma, lung squamous cell carcinoma, cervical squamous cell carcinoma, thyroid carcinoma, ovarian cancer, cancer of the rectum, pancreatic cancer, colon and stomach adenocarcinoma, uterine carcinosarcoma, and uterine corpus endometrial carcinoma, compared with normal control tissue but is downregulated in six types of cancer, including kidney carcinomas, acute myeloid leukemia, skin cutaneous melanoma, and testicular germ cell tumor. Furthermore, the authors used in silico tools to demonstrate specific expression within the central nervous system45. Elevated expression of TMPRSS4 correlates with poor prognosis of patients in various cancers (Table 1). Overexpression of TMPRSS4 is associated with stage progression and metastasis of multiple cancers, including colorectal46,47,48 and gastric cancers (Kim, unpublished results), suggesting a potential role for TMPRSS4 in the progression of noninvasive tumors to invasive malignancies.
The factors underlying TMPRSS4 upregulation and the mechanism(s) by which TMPRSS4 expression is regulated remain unclear. According to Villalba et al.49,50, high expression of TMPRSS4 protein in non-small cell lung cancer (NSCLC) patients is significantly associated with reduced relapse-free survival and overall survival. Bioinformatic analyses using public databases (COSMIC, CCLE, IGDB.NSCLC) show that in NSCLC, genetic alterations (i.e., gene amplification, mutations, and/or rearrangements) in TMPRSS4 are infrequent. Instead, aberrant hypomethylation in tumors correlates with high TMPRSS4 expression and is an independent prognostic predictor. Treatment of TMPRSS4-negative cells with a demethylating agent induces the expression of TMPRSS449. Although the TMPRSS4 promoter does not contain canonical CpG islands, it does contain relevant methylation regions, including CpGs located at positions −116 bp to +271 bp relative to the transcription start site (TSS). Loss of TMPRSS4 promoter methylation from CpGs spanning −116 bp to +271 bp relative to the TSS predicts a poor outcome for NSCLC patients. Analysis of transcription factor-binding site prediction databases suggests possible involvement of transcription factors related to proliferation, EMT, and inflammation; for example, binding of ZEB1, E2F1, Myc, NF-κB, and STAT3 close to these CpGs can be mediated by hypomethylation49, although further experimental studies are needed to determine how TMPRSS4 expression is modulated.
Furthermore, Villalba et al.50 showed that the expression of TMPRSS4 protein is an independent prognostic factor for NSCLC, particularly for patients with stage I cancer. The authors obtained plasma and bronchoalveolar lavage samples from healthy individuals and patients and measured the copy number of methylated and unmethylated CpGs within the TMPRSS4 promoter. They found that TMPRSS4 methylation status can be used as a diagnostic tool for early-stage patients and to monitor relapse in surgically resected patients. These results suggest that TMPRSS4 is a potential biomarker that can be used to identify patients at high risk of recurrence50. In addition, the hypomethylation status of TMPRSS4 could be used as a companion diagnostic marker for TMPRSS4-targeted therapy in the future.
In general, epigenetic changes occur at an early stage of tumor development. Compared with that in normal tissue, the expression of TMPRSS4 mRNA in atypical adenomatous hyperplasia, a known precursor for lung adenocarcinoma, is moderately (by 2-fold) upregulated, and it is further (by 8-fold) upregulated in early-stage lung adenocarcinoma, according to the data published by Sivakumar et al.51. Therefore, it is likely that epigenetic regulation may contribute to the upregulation of TMPRSS4 expression and that TMPRSS4 upregulation occurs at the premalignant and early stages of NSCLC. In contrast, overexpression of TMPRSS4 is associated with stage progression and metastasis of colorectal and gastric cancers. Consistent with this, analysis of TCGA datasets revealed that TMPRSS4 DNA is hypomethylated in certain cancer types, including NSCLC and pancreatic cancer, but not in colorectal or gastric cancers (Kim, unpublished results).
Expression of TMPRSS4 by NSCLC cells increases under hypoxic conditions52, suggesting that the hypoxic tumor microenvironment promotes TMPRSS4 expression. Consistent with this, a tripeptide, tyroserleutide, inhibits the irradiation-induced invasiveness and metastatic potential of HCC cells by downregulating HIF-1α and TMPRSS453. TMPRSS4 expression in NSCLC samples correlates negatively with that of tissue factor pathway inhibitor 2 (TFPI-2), and TFPI-2 partially inhibits transcription of TMPRSS4, leading to reduced lung cancer cell growth54. However, further investigations are needed. Several reports have shown that miRNAs or lncRNAs regulate TMPRSS4. For example, lncRNA HOXA11-AS acts as a tumor promoter in breast cancer by regulating the miR-125a-5p/TMPRSS4 axis55. miR-125a-p5, a key regulator in carcinogenesis that is expressed at abnormal levels by specific types of cancer, including NSCLC, colorectal, pancreatic, and prostate cancers, can target the 3′-UTR of TMPRSS4 mRNA to downregulate TMPRSS4 expression directly, resulting in reduced growth and enhanced apoptosis of lung cancer cells56. miR-1258, which plays an anticancer role in various cancers, can target TMPRSS4 directly and is associated with malignant progression of papillary thyroid carcinoma57. miR-541-3p can directly target and inhibit TMPRSS4 expression in HCC, thereby suppressing the invasion and migration of HCC cells58. miR-551b-3p, the expression of which is inhibited by lncRNA SMARCC2, functions as a tumor suppressor by directly suppressing TMPRSS4 expression, as well as the proliferation, motility and invasiveness of gastric cancer cells59.
Functions of TMPRSS4 during the regulation of EMT and CSCs
While the physiological function and in vivo substrates of TMPRSS4 remain unidentified, much of our knowledge about TMPRSS4 originates from clinical analysis and cell- and xenograft-based studies showing a correlation with cancer and other diseases. Indeed, a mutation in TMPRSS4 is associated with autosomal recessive cerebral atrophy syndrome, a novel pediatric neurodegenerative disorder60.
Knockdown of TMPRSS4 in lung, colon, and prostate cancer cells reduces cell migration and invasion through the ECM and suppresses proliferation, while overexpression increases cell migration and invasion of lung, colon, and prostate cancer cells and proliferation and anchorage-independent growth of lung and prostate cancer cells8,61,62. Reduction of TMPRSS4 expression in lung and prostate cancer cells inhibits tumor growth and metastasis in xenograft nude mouse models63,64, while overexpression of TMPRSS4 in lung, prostate, and colon cancer cells promotes tumor growth and metastasis in vivo62,64,65. In colon, prostate, and lung cancer cells, TMPRSS4 promotes the downregulation of E-cadherin, which is often accompanied by morphological changes and actin rearrangement, leading to EMT events and cancer cell invasion. TMPRSS4 also modulates cell-matrix adhesion and cell spreading by modulating integrins such as α5β1 and α4β1, which are implicated in EMT, cell motility, and/or cell survival8,64.
Among intracellular signaling mediators, TMPRSS4 activates the focal adhesion kinase (FAK)/c-Src, AKT, and ERK signaling pathways, mainly through upregulation of and possible interaction with integrin α5, leading to EMT and invasiveness. Consistent with this, expression of TMPRSS4 in human colorectal cancer tissues correlates positively with enhanced expression of integrin α5 and negatively with expression of E-cadherin, thereby confirming TMPRSS4-mediated regulation of EMT46. Larzabal et al.66 reported that miR-205 is involved in TMPRSS4-mediated upregulation of integrin α5 expression and metastasis of NSCLC cells, thereby supporting a link between TMPRSS4 and integrin α5. Cheng et al.67 reported that HAI-1 suppresses EMT in pancreatic cancer cells by modulating both matriptase and TMPRSS4, suggesting that TMPRSS4 activity may be regulated by the endogenous inhibitor HAI-1, although further investigation is needed to determine whether HAI-1 interacts with/inhibits TMPRSS4 directly. In addition, xenograft model studies have shown that TMPRSS4-mediated EMT plays a critical role in radiation-induced long-term metastasis of residual HCC68.
TMPRSS4 activates JNK and ERK1/2, leading to activation of the transcription factors activator protein-1 (AP-1) and SP1/361. TMPRSS4 also activates NF-κB in lung, prostate, and colon cancer cells, possibly through FAK- or AXL-mediated activation of AKT64,69. Consistent with this, TMPRSS4 promotes the invasion of gastric cancer cells70 and the growth of lung cancer cells56 by activating NF-κB signaling, although the precise mechanisms need to be determined. In addition, TMPRSS4 may be involved in regulating the tumor microenvironment (e.g., regulating the immune status or angiogenesis) via NF-κB, thereby contributing to malignancy; again, these possibilities require further investigation.
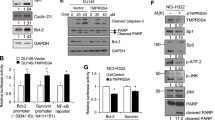
TMPRSS4 upregulates EMT-TFs such as ZEB2 (at the mRNA level), SLUG, and TWIST1 in a context/cell-dependent manner via MAPK-mediated activation of AP-1 and SP164. TMPRSS4 upregulates SLUG and cyclin D1 in prostate cancer cells by activating AP-1, leading to both invasion and proliferation. In addition, a positive feedback loop between SLUG and AP-1 promotes the expression of cyclin D1 and increases cell proliferation. SLUG activates AP-1 by upregulating AXL expression and signaling62. Consistent with this, TMPRSS4 promotes thyroid cancer cell proliferation via CREB phosphorylation and transcription of cyclin D171.
TMPRSS4 confers stem-like properties on prostate and colon cancer cells, inducing aldehyde dehydrogenase (ALDH) activation, tumorsphere formation, and resistance to chemotherapeutics and anoikis, thereby increasing the survival of circulating tumor cells and promoting early metastasis64. These features are accompanied by upregulation of the stemness-related factors SOX2, BMI1, CD133, and the ABC transporter MDR1/ABCB1. SOX2, a pluripotency-inducing transcription factor, is overexpressed in almost all human cancer types and is associated with a poor prognosis; it is also considered to be a key regulator of tumorigenicity, drug resistance, and metastasis72. SLUG and TWIST1 contribute to TMPRSS4-mediated CSC-like features through upregulation of SOX2. TWIST1 upregulates the transcription of SOX2 by interacting with the proximal E-box element in the SOX2 promoter, while SLUG binds to and stabilizes SOX2 to prevent proteasomal degradation. Clinically, expression of TMPRSS4 correlates with levels of ALDH, SOX2, PROM1, SNAI2, and TWIST1. Analysis of CCLE data reveals that SOX2 expression correlates positively with that of TWIST1 but not that of other EMT-inducing transcription factors64. TMPRSS4 contributes to tumor growth and metastatic seeding through diverse molecular mechanisms, thereby linking EMT and tumorigenic programs.
Similarly, De Aberasturi et al.73 reported that increased expression of CSC markers such as ALDH and OCT-4 correlates positively with expression of TMPRSS4 in NSCLC. In addition, overexpression of TMPRSS4 confers EMT features and CSC-like properties on lung cancer cells, accompanied by enhanced tumorigenicity in vivo. Overexpression of TMPRSS4 also causes cancer cells to become more resistant to chemotherapeutics73, while downregulation of TMPRSS4 significantly increases sensitivity to chemotherapeutics by impairing proliferation65. In addition, Villalba et al.74 recently performed large-scale analyses of five public NSCLC datasets and identified a synthetic lethal interaction between TMPRSS4 and DDR1; TMPRSS4/DDR double knockdown resulted in cell cycle arrest and apoptosis; furthermore, cells became highly sensitized to cisplatin in vitro, and tumors regressed in vivo.
Therefore, TMPRSS4 expression in a variety of different cancers is associated with poor prognosis and survival, which may be due to an increase in the CSC population. Further studies should investigate whether TMPRSS4 promotes tumor initiation in transgenic animal models.
Precursor substrates and signals regulated by TMPRSS4
Many studies have investigated the substrates of TTSPs. During tumor progression, TTSPs such as matriptase, hepsin, and TMPRSS2 activate pro-HGF to elicit the HGF/c-MET pro-oncogenic/cell survival signaling pathway in vivo and/or in vitro28,33. Matriptase and hepsin cleave and activate pro-MSP-1 and pro-uPA. Prostasin, a GPI-anchored protein, is also a substrate of matriptase and hepsin. The PAR-2/NF-κB inflammatory pathway is activated by matriptase, hepsin, and TMPRSS228.
When coexpressed with the mouse epithelial sodium channel (ENaC) in the Xenopus oocyte system, TMPRSS4, matriptase, and prostasin activate ENaC through proteolytic cleavage and subsequent removal of an inhibitory moiety from its γ-subunit, thereby regulating sodium and water flux across the high-resistance epithelium39,75. In zebrafish embryos, TMPRSS4 is necessary for organogenesis; indeed, knockdown of TMPRSS4 using morpholinos results in severe defects in tissue development and cell differentiation, including disturbed skeletal muscle formation, a decelerated heartbeat, and a degenerated vascular system76, suggesting that TMPRSS4 may modulate the activity of adhesion molecules involved in organ development. In contrast, TMPRSS4 knockout mice are viable and fertile and have no obvious abnormalities, suggesting functional redundancy of TMPRSS4 with respect to development and ENaC activation77. These data also suggest that targeted ablation of TMPRSS4 in cancer cells may have minimal side effects.
Similar to other TTSP family members, TMPRSS4 shows a preference for basic Lys and Arg residues at the P1 position, as shown by studies employing ENaC mutagenesis78 and focused peptide substrates8 (Kim, unpublished results). TMPRSS4 cleaves the γ-subunit of ENaC within a region of seven basic residues, thereby producing a unique ~70 kDa carboxyl-terminal fragment of the γ-subunit78. Regarding the precursor substrates cleaved by TMPRSS4 during tumor progression, Min et al.79 demonstrated that TMPRSS4 converts inactive pro-uPA into the active form directly through its serine proteolytic activity to promote cancer cell invasion. Consistent with this, TMPRSS4 promotes cancer cell invasion in a manner that is dependent on serine proteolytic activity79, and small-molecule compounds inhibiting TMPRSS4 serine protease activity reduce TMPRSS4-dependent invasion69. Furthermore, TMPRSS4 upregulates transcription of the uPA gene in prostate and lung cancer cells by activating AP-1 and SP1/3 in a MAPK (mainly JNK)-dependent manner. Expression of TMPRSS4 shows a strong positive correlation with uPA expression in human lung and prostate adenocarcinoma. In addition, TMPRSS4 interacts with the uPA receptor (uPAR/CD87) to activate the JNK, ERK, and c-Src signaling pathways in prostate and lung cancer cells61.
uPA is a well-known serine protease involved in invasion and metastasis; it catalyzes the conversion of inactive plasminogen into active plasmin, which can degrade most ECM components and activate MMPs to promote invasion. Increased levels of uPA correlate with a poor prognosis in many types of cancer, including breast, lung, stomach, bladder, colon, prostate, and ovarian cancers. Complexes of uPA and uPAR on the tumor cell surface interact with coreceptors (e.g., integrins) to activate intracellular signaling pathways, including MAPK, PI3K/AKT, Rac1, and JAK/STAT, which promote cell migration, invasion, survival, metastasis, EMT, stem cell-like properties, and chemotherapy resistance80. Both uPA and uPAR are involved in TMPRSS4-mediated invasion. TMPRSS4 activates the JNK, ERK, and c-Src signaling pathways, possibly through its interaction with uPAR, leading to activation of AP-1 and SP1 and subsequent expression of uPA. These results suggest that TMPRSS4 acts as an upstream regulator of the uPA/uPAR system by upregulating pro-uPA at both the transcriptional and posttranslational levels. The association between TMPRSS4 and uPAR and the subsequent modulation of cell signaling may be a novel mechanism controlling EMT, invasion, CSCs, and drug resistance. In the future, substrates of TMPRSS4 other than pro-uPA should be investigated both in vivo and in vitro. In addition, it would be worth investigating whether TMPRSS4 has a pro-oncogenic function and activates signaling pathways in a protease activity-independent manner.
Recently, Gu et al.81 showed that TMPRSS4 promotes cell proliferation and inhibits apoptosis in pancreatic ductal adenocarcinoma by activating the ERK1/2 signaling pathway, suggesting a pro-oncogenic role for TMPRSS4. Dong et al.82 showed that TMPRSS4 increases the expression of the heparin-binding-EGF precursor and promotes its proteolytic cleavage by enhancing MMP-9 expression through EGFR/AKT/mTOR/HIF-1α signaling, leading to angiogenesis, proliferation, and invasion in HCC.
Role of TMPRSS4 in viral infections and lung fibrosis
According to GTEx RNA-seq data, three TTSPs (TMPRSS2, matriptase, and hepsin) are expressed at high levels in healthy human lungs, whereas TMPRSS3 and TMPRSS13 are expressed at lower levels, and other TTSPs, including TMPRSS4, are expressed at very low levels or not at all34. At present, the levels of TMPRSS4 protein in normal tissues, including lung, remain to be determined. However, Zang et al.83 showed that TMPRSS2, matriptase, and TMPRSS4 are highly expressed in human intestinal epithelial cells, and TMPRSS4 and TMPRSS2 (but not matriptase) promote entry of SARS-CoV-2 into human small intestinal enterocytes. TMPRSS4 cleaves the spike protein, although less efficiently than TMPRSS2, in vitro83. In addition, TMPRSS2 and TMPRSS4 play a role in the spread of influenza virus by cleaving and activating HA. Transient expression of TMPRSS2 and TMPRSS4 in host cells facilitates cleavage of HA and replication of influenza virus in vitro43. Consistent with this, TMPRSS2 and TMPRSS4 double-knockout mice show a marked reduction in the spread of H3N2 influenza virus, whereas deletion of TMPRSS4 or TMPRSS2 alone does not or only slightly protects them from death upon infection with H3N2 influenza virus, although TMPRSS2-deficient mice are protected against H1N1 influenza virus infection84. Additionally, TMPRSS4 is strongly upregulated in the lungs of mice infected with H1N1 influenza virus85.
Among all TTSPs, matriptase and TMPRSS4 may play profibrotic roles in the lung that could contribute to the pathogenesis of idiopathic pulmonary fibrosis (IPF)34. TMPRSS4 is upregulated in the lungs of IPF patients and in a bleomycin-induced pulmonary fibrosis mouse model. TMPRSS4 is upregulated and expressed mainly by mast cells and alveolar epithelial cells, primarily in fibrotic areas of IPF lungs. Furthermore, TMPRSS4 deficiency attenuates bleomycin-induced lung fibrosis in mice86. However, the mechanisms underlying the profibrotic activity of TMPRSS4 are unclear.
Development of TMPRSS4 inhibitors
Modulating TMPRSS4 activity may be a treatment for several types of cancer. Screening of a small-molecule compound library identified 2-hydroxydiarylamide derivatives that inhibit TMPRSS4 serine protease activity in biochemical assays using a recombinant TMPRSS4 serine protease and a fluorogenic substrate87. Among these derivatives, IMD-0354, a selective IκB kinase (IKK)-β inhibitor that is effective in acute and subacute inflammatory disease, displayed an IC50 of 11 µM, and KRT1853, a novel derivative of IMD-0354, displayed twofold stronger inhibition87. IMD-0354 and KRT1853 inhibited the effects of TMPRSS4-mediated cellular signaling, including activation of SP1, AP-1, and NF-κB and induction of bcl-2 and survivin. Both compounds efficiently reduced cancer cell invasion and proliferation and induced apoptosis, although the possibility that the compounds modulate the activity of other serine proteases cannot be ruled out. Importantly, KRT1853 efficiently suppressed tumor growth in nude mice bearing prostate and colon cancer xenografts69. At present, the crystal structure of TMPRSS4 is not available. When it becomes available, it would be worth investigating the structure of the TMPRSS4 inhibitor-docking protease domain. In terms of drug repurposing, KRT1853 and IMD-0354 may be useful anticancer agents or good bases for further development.
Concluding remarks
Figure 1 summarizes the biological roles of TMPRSS4 in physiological and pathological contexts. Based on the expression patterns, biological roles, and structural characteristics of TMPRSS4, this druggable protease could be a novel therapeutic target in solid tumors. However, more studies of the functions and mechanisms are needed. In addition, genetically engineered mouse models are needed to fully unravel the functions of the signaling pathways summarized in this review. The TMPRSS4 knockout mice generated by Keppner et al.77 will be useful for future cancer studies. Due to the high similarity of the active sites among different serine proteases, it will be a challenge to develop a highly selective inhibitor of TMPRSS4 targeting the active site.
After being synthesized and translocated to the plasma membrane, TMPRSS4 is autoactivated by cleavage between R204 and V205. In the Xenopus oocyte system, TMPRSS4 activates ENaC by removing an inhibitory peptide of the γ-subunit. However, TMPRSS4-deficient mice show no abnormalities, suggesting functional redundancy in development, homeostasis, and ENaC activation. Under pathological conditions, TMPRSS4 cleaves the HA molecule of influenza virus to promote viral spread in the lung; it can also cleave the spike protein of SARS-CoV-2 to promote viral entry into intestinal enterocytes. Clinically, TMPRSS4 is a prognostic marker for diverse types of cancer and exhibits protumorigenic and prometastatic activity. The underlying mechanisms include acquisition of EMT and CSC-like properties, which confer chemotherapy resistance. TMPRSS4 cleaves pro-uPA directly to yield active uPA, thereby acting as an upstream regulator of the uPA/uPAR system. TMPRSS4 is upregulated in IPF lungs and in a bleomycin-induced pulmonary fibrosis mouse model. The fibrotic response in TMPRSS4-deficient mice is attenuated, suggesting that TMPRSS4 may play a profibrotic role. However, the underlying mechanisms remain unknown. LDLA: low-density lipoprotein receptor class A domain; SRCR: scavenger receptor cysteine-rich domain.
References
Lu, W. & Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell. 49, 361–374 (2019).
Yang, J. et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 21, 341–352 (2020).
Goossens, S., Vandamme, N., Van Vlierberghe, P. & Berx, G. EMT transcription factors in cancer development re-evaluated: Beyond EMT and MET. Biochim. Biophys. Acta Rev. Cancer. 1868, 584–591 (2017).
Dongre, A. & Weinberg, R. A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 20, 69–84 (2019).
Thiery, J. P., Acloque, H., Huang, R. Y. & Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell 139, 871–890 (2009).
Lambert, A. W. & Weinberg, R. A. Linking EMT programmes to normal and neoplastic epithelial stem cells. Nat. Rev. Cancer. 21, 325–338 (2021).
Mitschke, J., Burk, U. C. & Reinheckel, T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev. 38, 431–444 (2019).
Jung, H. et al. TMPRSS4 promotes invasion, migration and metastasis of human tumor cells by facilitating an epithelial-mesenchymal transition. Oncogene 27, 2635–2647 (2008).
Wang, C. H. et al. TMPRSS4 facilitates epithelial-mesenchymal transition of hepatocellular carcinoma and is a predictive marker for poor prognosis of patients after curative resection. Sci. Rep. 5, 12366 (2015).
Li, S. L. et al. Knockdown of TMPRSS3 inhibits gastric cancer cell proliferation, invasion and EMT via regulation of the ERK1/2 and PI3K/Akt pathways. Biomed. Pharmacother. 107, 841–848 (2018).
Lucas, J. M. et al. The androgen-regulated protease TMPRSS2 activates a proteolytic cascade involving components of the tumor microenvironment and promotes prostate cancer metastasis. Cancer Discov. 4, 1310–1325 (2014).
Lehmann, W. et al. ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types. Nat. Commun. 7, 10498 (2016).
Nam, E. H., Lee, Y., Park, Y. K., Lee, J. W. & Kim, S. ZEB2 upregulates integrin alpha5 expression through cooperation with Sp1 to induce invasion during epithelial-mesenchymal transition of human cancer cells. Carcinogenesis 33, 563–571 (2012).
Ko, D. & Kim, S. Cooperation between ZEB2 and Sp1 promotes cancer cell survival and angiogenesis during metastasis through induction of survivin and VEGF. Oncotarget 9, 726–742 (2018).
Wellner, U. et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat. Cell Biol. 11, 1487–1495 (2009).
Yang, M. H. et al. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat. Cell Biol. 12, 982–992 (2010).
Lee, D. et al. Interaction of tetraspan(in) TM4SF5 with CD44 promotes self-renewal and circulating capacities of hepatocarcinoma cells. Hepatology 61, 1978–1997 (2015).
Storci, G. et al. The basal-like breast carcinoma phenotype is regulated by SLUG gene expression. J. Pathol. 214, 25–37 (2008).
Luanpitpong, S. et al. SLUG is required for SOX9 stabilization and functions to promote cancer stem cells and metastasis in human lung carcinoma. Oncogene 35, 2824–2833 (2016).
Shibue, T. & Weinberg, R. A. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 14, 611–629 (2017).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Rawlings, N. D. et al. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 46, D624–D632 (2018).
Jiang, L., Yuan, C. & Huang, M. A general strategy to inhibit serine protease by targeting its autolysis loop. FASEB J. 35, e21259 (2021).
Drag, M. & Salvesen, G. S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Disco. 9, 690–701 (2010).
Turk, B. Targeting proteases: successes, failures and future prospects. Nat. Rev. Drug Disco. 5, 785–799 (2006).
Martin, C. E. & List, K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 38, 357–387 (2019).
Kim, S. & Lee, J. W. Membrane Proteins Involved in Epithelial-Mesenchymal Transition and Tumor Invasion: Studies on TMPRSS4 and TM4SF5. Genomics Inf. 12, 12–20 (2014).
Tanabe, L. M. & List, K. The role of type II transmembrane serine protease-mediated signaling in cancer. FEBS J. 284, 1421–1436 (2017).
Bugge, T. H., Antalis, T. M. & Wu, Q. Type II transmembrane serine proteases. J. Biol. Chem. 284, 23177–23181 (2009).
Ohler, A. & Becker-Pauly, C. TMPRSS4 is a type II transmembrane serine protease involved in cancer and viral infections. Biol. Chem. 393, 907–914 (2012).
Afar, D. E. et al. Catalytic cleavage of the androgen-regulated TMPRSS2 protease results in its secretion by prostate and prostate cancer epithelia. Cancer Res. 61, 1686–1692 (2001).
Kataoka, H., Kawaguchi, M., Fukushima, T. & Shimomura, T. Hepatocyte growth factor activator inhibitors (HAI-1 and HAI-2): Emerging key players in epithelial integrity and cancer. Pathol. Int. 68, 145–158 (2018).
Murray, A. S., Varela, F. A. & List, K. Type II transmembrane serine proteases as potential targets for cancer therapy. Biol. Chem. 397, 815–826 (2016).
Murza, A. et al. Inhibitors of type II transmembrane serine proteases in the treatment of diseases of the respiratory tract - A review of patent literature. Expert Opin. Ther. Pat. 30, 807–824 (2020).
Webb, S. L., Sanders, A. J., Mason, M. D. & Jiang, W. G. Type II transmembrane serine protease (TTSP) deregulation in cancer. Front. Biosci. (Landmark Ed.) 16, 539–552 (2011).
List, K. et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 19, 1934–1950 (2005).
Zoratti, G. L. et al. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat. Commun. 6, 6776 (2015).
Zuo, K. et al. Specifically targeting cancer proliferation and metastasis processes: the development of matriptase inhibitors. Cancer Metastasis Rev. 38, 507–524 (2019).
Szabo, R. & Bugge, T. H. Membrane-anchored serine proteases as regulators of epithelial function. Biochem. Soc. Trans. 48, 517–528 (2020).
Laporte, M. & Naesens, L. Airway proteases: an emerging drug target for influenza and other respiratory virus infections. Curr. Opin. Virol. 24, 16–24 (2017).
Oubahmane, M. et al. Host Cell Proteases Mediating SARS-CoV-2 Entry: An Overview. Curr. Top. Med. Chem. 22, 1776–1792 (2022).
Kim, T. S., Heinlein, C., Hackman, R. C. & Nelson, P. S. Phenotypic analysis of mice lacking the Tmprss2-encoded protease. Mol. Cell. Biol. 26, 965–975 (2006).
Bertram, S. et al. TMPRSS2 and TMPRSS4 facilitate trypsin-independent spread of influenza virus in Caco-2 cells. J. Virol. 84, 10016–10025 (2010).
Wallrapp, C. et al. A novel transmembrane serine protease (TMPRSS3) overexpressed in pancreatic cancer. Cancer Res. 60, 2602–2606 (2000).
Katopodis, P. et al. COVID19 and SARSCoV2 host cell entry mediators: Expression profiling of TMRSS4 in health and disease. Int. J. Mol. Med. 47, 64 (2021).
Kim, S. et al. TMPRSS4 induces invasion and epithelial-mesenchymal transition through upregulation of integrin alpha5 and its signaling pathways. Carcinogenesis 31, 597–606 (2010).
Huang, A. et al. TMPRSS4 correlates with colorectal cancer pathological stage and regulates cell proliferation and self-renewal ability. Cancer Biol. Ther. 15, 297–304 (2014).
Zhao, X. F., Yang, Y. S., Gao, D. Z. & Park, Y. K. TMPRSS4 overexpression promotes the metastasis of colorectal cancer and predicts poor prognosis of stage III-IV colorectal cancer. Int. J. Biol. Markers. 36, 23–32 (2021).
Villalba, M. et al. Epigenetic alterations leading to TMPRSS4 promoter hypomethylation and protein overexpression predict poor prognosis in squamous lung cancer patients. Oncotarget 7, 22752–22769 (2016).
Villalba, M. et al. TMPRSS4: A Novel Tumor Prognostic Indicator for the Stratification of Stage IA Tumors and a Liquid Biopsy Biomarker for NSCLC Patients. J. Clin. Med. 8, 2134 (2019).
Sivakumar, S. et al. Genomic Landscape of Atypical Adenomatous Hyperplasia Reveals Divergent Modes to Lung Adenocarcinoma. Cancer Res. 77, 6119–6130 (2017).
Nguyen, T. H. et al. Expression of TMPRSS4 in non-small cell lung cancer and its modulation by hypoxia. Int. J. Oncol. 41, 829–838 (2012).
Jia, J. B. et al. A novel tripeptide, tyroserleutide, inhibits irradiation-induced invasiveness and metastasis of hepatocellular carcinoma in nude mice. Invest. N. Drugs. 29, 861–872 (2011).
Hamamoto, J. et al. Methylation-induced downregulation of TFPI-2 causes TMPRSS4 overexpression and contributes to oncogenesis in a subset of non-small-cell lung carcinoma. Cancer Sci. 106, 34–42 (2015).
Xu, Y., Ren, Z., Wang, X. & Ren, M. The lncRNA HOXA11-AS acts as a tumor promoter in breast cancer through regulation of the miR-125a-5p/TMPRSS4 axis. J. Gene Med. 24, e3413 (2022).
Fan, X. et al. The upregulation of TMPRSS4, partly ascribed to the downregulation of miR125a5p, promotes the growth of human lung adenocarcinoma via the NFkappaB signaling pathway. Int. J. Oncol. 53, 148–158 (2018).
Wang, L. J. & Cai, H. Q. miR-1258: a novel microRNA that controls TMPRSS4 expression is associated with malignant progression of papillary thyroid carcinoma. Endokrynol. Pol. 71, 146–152 (2020).
Xia, Y. H., Ren, L., Li, J. Z. & Gao, F. Role of miR-541-3p/TMPRSS4 in the metastasis and EMT of hepatocellular carcinoma. Eur. Rev. Med. Pharmacol. Sci. 23, 10721–10728 (2019).
Yuan, H. et al. Molecular mechanisms of lncRNA SMARCC2/miR-551b-3p/TMPRSS4 axis in gastric cancer. Cancer Lett. 418, 84–96 (2018).
Lahiry, P. et al. A mutation in the serine protease TMPRSS4 in a novel pediatric neurodegenerative disorder. Orphanet J. Rare Dis. 8, 126 (2013).
Min, H. J. et al. TMPRSS4 upregulates uPA gene expression through JNK signaling activation to induce cancer cell invasion. Cell. Signal. 26, 398–408 (2014).
Lee, Y. et al. TMPRSS4 induces invasion and proliferation of prostate cancer cells through induction of Slug and cyclin D1. Oncotarget 7, 50315–50332 (2016).
Larzabal, L. et al. Overexpression of TMPRSS4 in non-small cell lung cancer is associated with poor prognosis in patients with squamous histology. Br. J. Cancer. 105, 1608–1614 (2011).
Lee, Y. et al. TMPRSS4 promotes cancer stem-like properties in prostate cancer cells through upregulation of SOX2 by SLUG and TWIST1. J. Exp. Clin. Cancer Res. 40, 372 (2021).
Exposito, F. et al. Targeting of TMPRSS4 sensitizes lung cancer cells to chemotherapy by impairing the proliferation machinery. Cancer Lett. 453, 21–33 (2019).
Larzabal, L. et al. TMPRSS4 regulates levels of integrin alpha5 in NSCLC through miR-205 activity to promote metastasis. Br. J. Cancer. 110, 764–774 (2014).
Cheng, H., Fukushima, T., Takahashi, N., Tanaka, H. & Kataoka, H. Hepatocyte growth factor activator inhibitor type 1 regulates epithelial to mesenchymal transition through membrane-bound serine proteinases. Cancer Res. 69, 1828–1835 (2009).
Li, T. et al. Radiation enhances long-term metastasis potential of residual hepatocellular carcinoma in nude mice through TMPRSS4-induced epithelial-mesenchymal transition. Cancer Gene Ther. 18, 617–626 (2011).
Kim, S. et al. Anti-cancer activity of the novel 2-hydroxydiarylamide derivatives IMD-0354 and KRT1853 through suppression of cancer cell invasion, proliferation, and survival mediated by TMPRSS4. Sci. Rep. 9, 10003 (2019).
Jin, J., Shen, X., Chen, L., Bao, L. W. & Zhu, L. M. TMPRSS4 promotes invasiveness of human gastric cancer cells through activation of NF-kappaB/MMP-9 signaling. Biomed. Pharmacother. 77, 30–36 (2016).
Guan, H. et al. Transmembrane Protease Serine 4 Promotes Thyroid Cancer Proliferation via CREB Phosphorylation. Thyroid 25, 85–94 (2015).
Novak, D. et al. SOX2 in development and cancer biology. Semin. Cancer Biol. 67, 74–82 (2020).
de Aberasturi, A. L. et al. TMPRSS4 induces cancer stem cell-like properties in lung cancer cells and correlates with ALDH expression in NSCLC patients. Cancer Lett. 370, 165–176 (2016).
Villalba, M. et al. Identification of a novel synthetic lethal vulnerability in non-small cell lung cancer by co-targeting TMPRSS4 and DDR1. Sci. Rep. 9, 15400 (2019).
Andreasen, D., Vuagniaux, G., Fowler-Jaeger, N., Hummler, E. & Rossier, B. C. Activation of epithelial sodium channels by mouse channel activating proteases (mCAP) expressed in Xenopus oocytes requires catalytic activity of mCAP3 and mCAP2 but not mCAP1. J. Am. Soc. Nephrol. 17, 968–976 (2006).
Ohler, A. & Becker-Pauly, C. Morpholino knockdown of the ubiquitously expressed transmembrane serine protease TMPRSS4a in zebrafish embryos exhibits severe defects in organogenesis and cell adhesion. Biol. Chem. 392, 653–664 (2011).
Keppner, A. et al. Epithelial Sodium Channel-Mediated Sodium Transport Is Not Dependent on the Membrane-Bound Serine Protease CAP2/Tmprss4. PLoS One. 10, e0135224 (2015).
Passero, C. J. et al. TMPRSS4-dependent activation of the epithelial sodium channel requires cleavage of the gamma-subunit distal to the furin cleavage site. Am. J. Physiol. Ren. Physiol. 302, F1–F8 (2012).
Min, H. J., Lee, M. K., Lee, J. W. & Kim, S. TMPRSS4 induces cancer cell invasion through pro-uPA processing. Biochem. Biophys. Res. Commun. 446, 1–7 (2014).
Gonias, S. L. & Hu, J. Urokinase receptor and resistance to targeted anticancer agents. Front. Pharmacol. 6, 154 (2015).
Gu, J. et al. TMPRSS4 Promotes Cell Proliferation and Inhibits Apoptosis in Pancreatic Ductal Adenocarcinoma by Activating ERK1/2 Signaling Pathway. Front. Oncol. 11, 628353 (2021).
Dong, Z. R. et al. TMPRSS4 Drives Angiogenesis in Hepatocellular Carcinoma by Promoting HB-EGF Expression and Proteolytic Cleavage. Hepatology 72, 923–939 (2020).
Zang, R. et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 5, 47 (2020).
Kuhn, N. et al. The Proteolytic Activation of (H3N2) Influenza A Virus Hemagglutinin Is Facilitated by Different Type II Transmembrane Serine Proteases. J. Virol. 90, 4298–4307 (2016).
Bahgat, M. M., Blazejewska, P. & Schughart, K. Inhibition of lung serine proteases in mice: a potentially new approach to control influenza infection. Virol. J. 8, 27 (2011).
Valero-Jimenez, A. et al. Transmembrane protease, serine 4 (TMPRSS4) is upregulated in IPF lungs and increases the fibrotic response in bleomycin-induced lung injury. PLoS One. 13, e0192963 (2018).
Kang, S., Min, H. J., Kang, M. S., Jung, M. G. & Kim, S. Discovery of novel 2-hydroxydiarylamide derivatives as TMPRSS4 inhibitors. Bioorg. Med. Chem. Lett. 23, 1748–1751 (2013).
Cheng, D., Kong, H. & Li, Y. TMPRSS4 as a poor prognostic factor for triple-negative breast cancer. Int. J. Mol. Sci. 14, 14659–14668 (2013).
Li, X. M. et al. Overexpression of TMPRSS4 promotes tumor proliferation and aggressiveness in breast cancer. Int. J. Mol. Med. 39, 927–935 (2017).
Liang, B., Wu, M., Bu, Y., Zhao, A. & Xie, F. Prognostic value of TMPRSS4 expression in patients with breast cancer. Med. Oncol. 30, 497 (2013).
Luo, Z. Y., Wang, Y. Y., Zhao, Z. S., Li, B. & Chen, J. F. The expression of TMPRSS4 and Erk1 correlates with metastasis and poor prognosis in Chinese patients with gastric cancer. PLoS One. 8, e70311 (2013).
Tazawa, H. et al. Utility of TMPRSS4 as a Prognostic Biomarker and Potential Therapeutic Target in Patients with Gastric Cancer. J. Gastrointest. Surg. 26, 305–313 (2022).
Sheng, H., Shen, W., Zeng, J., Xi, L. & Deng, L. Prognostic significance of TMPRSS4 in gastric cancer. Neoplasma 61, 213–217 (2014).
Wu, X. Y. et al. Clinical implication of TMPRSS4 expression in human gallbladder cancer. Tumour Biol. 35, 5481–5486 (2014).
Cheng, D., Liang, B. & Li, Y. High TMPRSS4 expression is a predictor of poor prognosis in cervical squamous cell carcinoma. Cancer Epidemiol. 37, 993–997 (2013).
Huang, A. et al. High expression level of TMPRSS4 predicts adverse outcomes of colorectal cancer patients. Med. Oncol. 30, 712 (2013).
Acknowledgements
The author thanks D.K., J.Y., and Y.L. for assistance with dataset analysis. This study was supported by grants from the Korea Drug Development Fund funded by the Ministry of Science and ICT, Ministry of Trade, Industry, and Energy and the Ministry of Health and Welfare (RS-2022-00166100), the National Research Foundation of Korea (NRF-2020R1A2C1008796), and Yuhan Corporation, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, S. TMPRSS4, a type II transmembrane serine protease, as a potential therapeutic target in cancer. Exp Mol Med 55, 716–724 (2023). https://doi.org/10.1038/s12276-023-00975-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s12276-023-00975-5