Abstract
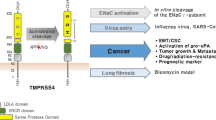
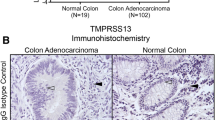
Breast cancer progression is accompanied by increased expression of extracellular and cell-surface proteases capable of degrading the extracellular matrix as well as cleaving and activating downstream targets. The type II transmembrane serine proteases (TTSPs) are a family of cell-surface proteases that play critical roles in numerous types of cancers. Therefore, the aim of this study was to identify novel and uncharacterized TTSPs with differential expression in breast cancer and to determine their potential roles in progression. Systematic in silico data analysis followed by immunohistochemical validation identified increased expression of the TTSP family member, TMPRSS13 (transmembrane protease, serine 13), in invasive ductal carcinoma patient tissue samples compared to normal breast tissue. To test whether loss of TMPRSS13 impacts tumor progression, TMPRSS13 was genetically ablated in the oncogene-induced transgenic MMTV-PymT tumor model. TMPRSS13 deficiency resulted in a significant decrease in overall tumor burden and growth rate, as well as a delayed formation of detectable mammary tumors, thus suggesting a causal relationship between TMPRSS13 expression and the progression of breast cancer. Complementary studies using human breast cancer cell culture models revealed that siRNA-mediated silencing of TMPRSS13 expression decreases proliferation, induces apoptosis, and attenuates invasion. Importantly, targeting TMPRSS13 expression renders aggressive triple-negative breast cancer cell lines highly responsive to chemotherapy. At the molecular level, knockdown of TMPRSS13 in breast cancer cells led to increased protein levels of the tumor-suppressive protease prostasin. TMPRSS13/prostasin co-immunoprecipitation and prostasin zymogen activation experiments identified prostasin as a potential novel target for TMPRSS13. Regulation of prostasin levels may be a mechanism that contributes to the pro-oncogenic properties of TMPRSS13 in breast cancer. TMPRSS13 represents a novel candidate for targeted therapy in combination with standard of care chemotherapy agents in patients with hormone receptor-negative breast cancer or in patients with tumors refractory to endocrine therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Hooper JD, Clements JA, Quigley JP, Antalis TM. Type II transmembrane serine proteases. Insights into an emerging class of cell surface proteolytic enzymes. J Biol Chem. 2001;276:857–60.
Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, Bugge TH, et al. Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev. 2003;22:237–58.
Antalis TM, Buzza MS, Hodge KM, Hooper JD, Netzel-Arnett S. The cutting edge: membrane-anchored serine protease activities in the pericellular microenvironment. Biochemical J. 2010;428:325–46.
Antalis TM, Bugge TH, Wu Q. Membrane-anchored serine proteases in health and disease. Prog Mol Biol Transl Sci. 2011;99:1–50.
Bugge TH, Antalis TM, Wu Q. Type II transmembrane serine proteases. J Biol Chem. 2009;284:23177–81.
List K, Bugge TH, Szabo R. Matriptase: potent proteolysis on the cell surface. Mol Med. 2006;12:1–7.
Martin CE, List K. Cell surface-anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019;38:357–87.
Murray AS, Varela FA, List K. Type II transmembrane serine proteases as potential targets for cancer therapy. Biol Chem. 2016;397:815–26.
Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40:1297–316.
Tanabe LM, List K. The role of type II transmembrane serine protease-mediated signaling in cancer. Febs J. 2017;284:1421–36.
Kido H, Okumura Y. MSPL/TMPRSS13. Front Biosci. 2008;13:754–8.
Okumura Y, Takahashi E, Yano M, Ohuchi M, Daidoji T, Nakaya T, et al. Novel type II transmembrane serine proteases, MSPL and TMPRSS13, Proteolytically activate membrane fusion activity of the hemagglutinin of highly pathogenic avian influenza viruses and induce their multicycle replication. J Virol. 2010;84:5089–96.
Zmora P, Blazejewska P, Moldenhauer AS, Welsch K, Nehlmeier I, Wu Q, et al. DESC1 and MSPL activate influenza A viruses and emerging coronaviruses for host cell entry. J Virol. 2014;88:12087–97.
Hashimoto T, Kato M, Shimomura T, Kitamura N. TMPRSS13, a type II transmembrane serine protease, is inhibited by hepatocyte growth factor activator inhibitor type 1 and activates pro-hepatocyte growth factor. Febs J. 2010;277:4888–4900.
Madsen DH, Szabo R, Molinolo AA, Bugge TH. TMPRSS13 deficiency impairs stratum corneum formation and epidermal barrier acquisition. Biochem J. 2014;461:487–95.
List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19:1934–50.
Zoratti GL, Tanabe LM, Varela FA, Murray AS, Bergum C, Colombo E, et al. Targeting matriptase in breast cancer abrogates tumour progression via impairment of stromal-epithelial growth factor signalling. Nat Commun. 2015;6:6776.
Bao Y, Li K, Guo Y, Wang Q, Li Z, Yang Y, et al. Tumor suppressor PRSS8 targets Sphk1/S1P/Stat3/Akt signaling in colorectal cancer. Oncotarget. 2016;7:26780–92.
Bao Y, Wang Q, Guo Y, Chen Z, Li K, Yang Y, et al. PRSS8 methylation and its significance in esophageal squamous cell carcinoma. Oncotarget. 2016;7:28540–55.
Bao Y, Guo Y, Yang Y, Wei X, Zhang S, Zhang Y, et al. PRSS8 suppresses colorectal carcinogenesis and metastasis. Oncogene. 2019;38:497–517.
Chen LM, Hodge GB, Guarda LA, Welch JL, Greenberg NM, Chai KX. Down-regulation of prostasin serine protease: a potential invasion suppressor in prostate cancer. Prostate. 2001;48:93–103.
Chen LM, Chai KX. Prostasin serine protease inhibits breast cancer invasiveness and is transcriptionally regulated by promoter DNA methylation. Int J Cancer. 2002;97:323–9.
Chen LM, Verity NJ, Chai KX. Loss of prostasin (PRSS8) in human bladder transitional cell carcinoma cell lines is associated with epithelial-mesenchymal transition (EMT). BMC Cancer. 2009;9:377.
Yamamoto K, Kawaguchi M, Shimomura T, Izumi A, Konari K, Honda A, et al.Hepatocyte growth factor activator inhibitor type-2 (HAI-2)/SPINT2 contributes to invasive growth of oral squamous cell carcinoma cells. Oncotarget. 2018;9:11691–706.
Yan BX, Ma JX, Zhang J, Guo Y, Mueller MD, Remick SC, et al. Prostasin may contribute to chemoresistance, repress cancer cells in ovarian cancer, and is involved in the signaling pathways of CASP/PAK2-p34/actin. Cell Death Dis. 2014;5:e995.
Goswami CP, Nakshatri H. PROGgeneV2: enhancements on the existing database. BMC Cancer. 2014;14:970.
Pawitan Y, Bjohle J, Amler L, Borg AL, Egyhazi S, Hall P, et al. Gene expression profiling spares early breast cancer patients from adjuvant therapy: derived and validated in two population-based cohorts. Breast Cancer Res. 2005;7:R953–964.
Murray AS, Varela FA, Hyland TE, Schoenbeck AJ, White JM, Tanabe LM. et al. Phosphorylation of the type II transmembrane serine protease, TMPRSS13 in hepatocyte growth factor activator Inhibitor-1 and 2-mediated cell surface localization.J Biolog Chem. 2017;292:14867–84.
Guy CT, Cardiff RD, Muller WJ. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol Cell Biol. 1992;12:954–61.
Lin EY, Jones JG, Li P, Zhu L, Whitney KD, Muller WJ, et al. Progression to malignancy in the polyoma middle T oncoprotein mouse breast cancer model provides a reliable model for human diseases. Am J Pathol. 2003;163:2113–26.
Hollestelle A, Nagel JH, Smid M, Lam S, Elstrodt F, Wasielewski M, et al. Distinct gene mutation profiles among luminal-type and basal-type breast cancer cell lines. Breast Cancer Res Treat. 2010;121:53–64.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Chen LM, Zhang X, Chai KX. Regulation of prostasin expression and function in the prostate. Prostate. 2004;59:1–12.
Chen LM, Skinner ML, Kauffman SW, Chao J, Chao L, Thaler CD, et al. Prostasin is a glycosylphosphatidylinositol-anchored active serine protease. J Biol Chem. 2001;276:21434–42.
Yu JX, Chao L, Chao J. Prostasin is a novel human serine proteinase from seminal fluid. Purification, tissue distribution, and localization in prostate gland. J Biol Chem. 1994;269:18843–8.
Yu JX, Chao L, Chao J. Molecular cloning, tissue-specific expression, and cellular localization of human prostasin mRNA. J Biol Chem. 1995;270:13483–9.
Shipway A, Danahay H, Williams JA, Tully DC, Backes BJ, Harris JL. Biochemical characterization of prostasin, a channel activating protease. Biochem Biophys Res Commun. 2004;324:953–63.
Friis S, Uzzun Sales K, Godiksen S, Peters DE, Lin CY, Vogel LK, et al. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem. 2013;288:19028–39.
Chen LM, Wang C, Chen M, Marcello MR, Chao J, Chao L, et al. Prostasin attenuates inducible nitric oxide synthase expression in lipopolysaccharide-induced urinary bladder inflammation. Am J Physiol Ren Physiol. 2006;291:F567–577.
Netzel-Arnett S, Currie BM, Szabo R, Lin CY, Chen LM, Chai KX, et al. Evidence for a matriptase-prostasin proteolytic cascade regulating terminal epidermal differentiation. J Biol Chem. 2006;281:32941–5.
Wahba HA, El-Hadaad HA. Current approaches in treatment of triple-negative breast cancer. Cancer Biol Med. 2015;12:106–16.
Quinn JE, Kennedy RD, Mullan PB, Gilmore PM, Carty M, Johnston PG, et al. BRCA1 functions as a differential modulator of chemotherapy-induced apoptosis. Cancer Res. 2003;63:6221–8.
Tassone P, Tagliaferri P, Perricelli A, Blotta S, Quaresima B, Martelli ML, et al. BRCA1 expression modulates chemosensitivity of BRCA1-defective HCC1937 human breast cancer cells. Br J Cancer. 2003;88:1285–91.
Yang HY, Fang DZ, Ding LS, Hui XB, Liu D. Overexpression of protease serine 8 inhibits glioma cell proliferation, migration, and invasion via suppressing the Akt/mTOR signaling pathway. Oncol Res. 2017;25:923–30.
Carne Trecesson S, Souaze F, Basseville A, Bernard AC, Pecot J, Lopez J, et al. BCL-XL directly modulates RAS signalling to favour cancer cell stemness. Nat Commun. 2017;8:1123.
Amundson SA, Myers TG, Scudiero D, Kitada S, Reed JC, Fornace AJ Jr. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 2000;60:6101–10.
Wei G, Margolin AA, Haery L, Brown E, Cucolo L, Julian B, et al. Chemical genomics identifies small-molecule MCL1 repressors and BCL-xL as a predictor of MCL1 dependency. Cancer Cell. 2012;21:547–62.
Takahashi S, Suzuki S, Inaguma S, Ikeda Y, Cho YM, Hayashi N, et al. Down-regulated expression of prostasin in high-grade or hormone-refractory human prostate cancers. Prostate. 2003;54:187–93.
Selzer-Plon J, Bornholdt J, Friis S, Bisgaard HC, Lothe IM, Tveit KM, et al. Expression of prostasin and its inhibitors during colorectal cancer carcinogenesis. BMC cancer. 2009;9:201.
Sakashita K, Mimori K, Tanaka F, Tahara K, Inoue H, Sawada T, et al. Clinical significance of low expression of Prostasin mRNA in human gastric cancer. J Surg Oncol. 2008;98:559–64.
Chen M, Chen LM, Lin CY, Chai KX. The epidermal growth factor receptor (EGFR) is proteolytically modified by the Matriptase-Prostasin serine protease cascade in cultured epithelial cells. Biochimica et biophysica acta. 2008;1783:896–903.
Chen M, Chen LM, Lin CY, Chai KX. Hepsin activates prostasin and cleaves the extracellular domain of the epidermal growth factor receptor. Mol Cell Biochem. 2010;337:259–66.
Leyvraz C, Charles RP, Rubera I, Guitard M, Rotman S, Breiden B, et al. The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J Cell Biol. 2005;170:487–96.
Friis S, Madsen DH, Bugge TH.Distinct developmental functions of prostasin (CAP1/PRSS8) zymogen and activated prostasin. J Biol Chem. 2016;291:2577–82.
Peters DE, Szabo R, Friis S, Shylo NA, Uzzun Sales K, Holmbeck K, et al. The membrane-anchored serine protease prostasin (CAP1/PRSS8) supports epidermal development and postnatal homeostasis independent of its enzymatic activity.J Biol Chem. 2014;289:14740–9.
Chen M, Fu YY, Lin CY, Chen LM, Chai KX. Prostasin induces protease-dependent and independent molecular changes in the human prostate carcinoma cell line PC-3. Biochimica et biophysica acta. 2007;1773:1133–40.
Wang J, Xi Z, Xi J, Zhang H, Li J, Xia Y, et al. Somatic mutations in renal cell carcinomas from Chinese patients revealed by whole exome sequencing. Cancer Cell Int. 2018;18:159.
Dai X, Cheng H, Bai Z, Li J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J Cancer. 2017;8:3131–41.
List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168:1513–25.
Acknowledgements
This work was supported by NIH/NCI R01CA160565 grant (KL), NIH/NCI R01CA160565-04S grant (KL, FAV), NIH/NCI R01CA222359 (KL), NIH/NCI F31CA217148 (FAV), NIGMS/NIH grant R25 GM 058905-15 (FAV), NIH Ruth L. Kirschstein National Research Service Award T32-CA009531 (ASM and CEM) and The DeRoy Testamentary Foundation (ASM).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Murray, A.S., Hyland, T.E., Sala-Hamrick, K.E. et al. The cell-surface anchored serine protease TMPRSS13 promotes breast cancer progression and resistance to chemotherapy. Oncogene 39, 6421–6436 (2020). https://doi.org/10.1038/s41388-020-01436-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-020-01436-3