Abstract
Objective
BCORL1, a transcriptional co-repressor, has a role in cortical migration, neuronal differentiation, maturation, and cerebellar development. We describe BCORL1 as a new genetic cause for major brain malformations.
Methods and results
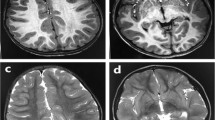
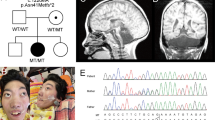
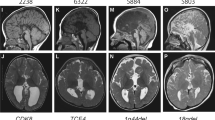
We report three patients from two unrelated families with neonatal onset intractable epilepsy and profound global developmental delay. Brain MRI of two siblings from the first family depicted hypoplastic corpus callosum and septal agenesis (ASP) in the older brother and unilateral perisylvian polymicrogyria (PMG) in the younger one. MRI of the patient from the second family demonstrated complete agenesis of corpus callosum (CC). Whole Exome Sequencing revealed a novel hemizygous variant in NM_021946.5 (BCORL1):c.796C>T (p.Pro266Ser) in the two siblings from the first family and the NM_021946.5 (BCORL1): c.3376G>A; p.Asp1126Asn variant in the patient from the second family, both variants inherited from healthy mothers. We reviewed the patients’ charts and MRIs and compared the phenotype to the other published BCORL1-related cases. Brain malformations have not been previously described in association with the BCORL1 phenotype. We discuss the potential influence of BCORL1 on brain development.
Conclusions
We suggest that BCORL1 variants present with a spectrum of neurodevelopmental disorders and can lead to major brain malformations originating at different stages of fetal development. We suggest adding BCORL1 to the genetic causes of PMG, ASP, and CC dysgenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Data available on request from the authors.
References
Schuurs-Hoeijmakers JH, Vulto-van Silfhout AT, Vissers LE, van de Vondervoort II, van Bon BW, de Ligt J, et al. Identification of pathogenic gene variants in small families with intellectually disabled siblings by exome sequencing. J Med Genet. 2013;50:802–11.
Shukla A, Girisha KM, Somashekar PH, Nampoothiri S, McClellan R, Vernon HJ. Variants in the transcriptional corepressor BCORL1 are associated with an X-linked disorder of intellectual disability, dysmorphic features, and behavioral abnormalities. Am J Med Genet A. 2019;179:870–4.
Jiang YH, Yuen RK, Jin X, Wang M, Chen N, Wu X, et al. Detection of clinically relevant genetic variants in autism spectrum disorder by whole-genome qsequencing. Am J Hum Genet. 2013;93:249–63.
Muthusamy B, Bellad A, Girimaji SC, Pandey A. Shukla-Vernon syndrome: a second family with a novel variant in the BCORL1 gene. Genes. 2021;12:452.
Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–21.
Yakovlev PI, Locke S. Limbic nuclei of thalamus and connections of limbic cortex. III. Corticocortical connections of the anterior cingulate gyrus, the cingulum, and the subcallosal bundle in monkey. Arch Neurol. 1961;5:364–400.
Yakovlev PI, Wadsworth RC. Schizencephalies; a study of the congenital clefts in the cerebral mantle; clefts with hydrocephalus and lips separated. J Neuropathol Exp Neurol. 1946;5:169–206.
Aicardi J, Goutières F. The syndrome of absence of the septum pellucidum with porencephalies and other developmental defects. Neuropediatrics. 1981;12:319–29.
Barkovich AJ, Norman D. Absence of the septum pellucidum: a useful sign in the diagnosis of congenital brain malformations. AJR Am J Roentgenol. 1989;152:353–60.
Schaefer GB, Bodensteiner JB, Thompson JN Jr, Kimberling WJ, Craft JM. Subtle anomalies of the septum pellucidum and neurodevelopmental deficits. Dev Med Child Neurol. 1994;36:554–9.
Malinger G, Lev D, Kidron D, Heredia F, Hershkovitz R, Lerman-Sagie T. Differential diagnosis in fetuses with absent septum pellucidum. Ultrasound Obstet Gynecol. 2005;25:42–9.
McCabe MJ, Alatzoglou KS, Dattani MT. Septo-optic dysplasia and other midline defects: the role of transcription factors: HESX1 and beyond. Best Pract Res Clin Endocrinol Metab. 2011;25:115–24.
Webb EA, Dattani MT. Septo-optic dysplasia. Eur J Hum Genet. 2010;18:393–7.
Kelberman D, Rizzoti K, Avilion A, Bitner-Glindzicz M, Cianfarani S, Collins J, et al. Mutations within Sox2/SOX2 are associated with abnormalities in the hypothalamo-pituitary-gonadal axis in mice and humans. J Clin Investig. 2006;116:2442–55.
Alatzoglou KS, Kelberman D, Dattani MT. The role of SOX proteins in normal pituitary development. J Endocrinol. 2009;200:245–58.
Ganau M, Huet S, Syrmos N, Meloni M, Jayamohan J. Neuro-ophthalmological manifestations of septo-optic dysplasia: current perspectives. Eye Brain. 2019;11:37–47.
Cohen RN, Cohen LE, Botero D, Yu C, Sagar A, Jurkiewicz M, et al. Enhanced repression by HESX1 as a cause of hypopituitarism and septooptic dysplasia. J Clin Endocrinol Metab. 2003;88:4832–9.
Judkins AR, Martinez D, Ferreira P, Dobyns WB, Golden JA. Polymicrogyria includes fusion of the molecular layer and decreased neuronal populations but normal cortical laminar organization. J Neuropathol Exp Neurol. 2011;70:438–43.
Leventer RJ, Jansen A, Pilz DT, Stoodley N, Marini C, Dubeau F, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–27.
Dvorak K, Feit J, Juránková Z. Experimentally induced focal microgyria and status verrucosus deformis in rats–pathogenesis and interrelation. Histological and autoradiographical study. Acta Neuropathol. 1978;44:121–9.
Choe Y, Siegenthaler JA, Pleasure SJ. A cascade of morphogenic signaling initiated by the meninges controls corpus callosum formation. Neuron. 2012;73:698–712.
Eriksson SH, Thom M, Heffernan J, Lin WR, Harding BN, Squier MV, et al. Persistent reelin-expressing Cajal-Retzius cells in polymicrogyria. Brain. 2001;124:1350–61.
Barkovich AJ. Current concepts of polymicrogyria. Neuroradiology. 2010;52:479–87.
Siegenthaler JA, Ashique AM, Zarbalis K, Patterson KP, Hecht JH, Kane MA, et al. Retinoic acid from the meninges regulates cortical neuron generation. Cell. 2009;139:597–609.
Squier W, Jansen A. Polymicrogyria: pathology, fetal origins and mechanisms. Acta Neuropathol Commun. 2014;2:80.
Zarbalis K, Choe Y, Siegenthaler JA, Orosco LA, Pleasure SJ. Meningeal defects alter the tangential migration of cortical interneurons in Foxc1hith/hith mice. Neural Dev. 2012;7:2.
López-Bendito G, Sánchez-Alcañiz JA, Pla R, Borrell V, Picó E, Valdeolmillos M, et al. Chemokine signaling controls intracortical migration and final distribution of GABAergic interneurons. J Neurosci. 2008;28:1613–24.
Siejka S, Strefling AM, Urich H. Absence of septum pellucidum and polymicrogyria: a forme fruste of the porencephalic syndrome. Clin Neuropathol. 1989;8:174–8.
Labate A, Gambardella A, Quattrone A. Septo-optic dysplasia plus bilateral perisylvian polymicrogyria: a case report. Neurol Sci. 2013;34:1479–80.
Becker PS, Dixon AM, Troncoso JC. Bilateral opercular polymicrogyria. Ann Neurol. 1989;25:90–2.
Mellado C, Poduri A, Gleason D, Elhosary PC, Barry BJ, Partlow JN, et al. Candidate gene sequencing of LHX2, HESX1, and SOX2 in a large schizencephaly cohort. Am J Med Genet A. 2010;152a:2736–42.
Pagan JK, Arnold J, Hanchard KJ, Kumar R, Bruno T, Jones MJ, et al. A novel corepressor, BCoR-L1, represses transcription through an interaction with CtBP. J Biol Chem. 2007;282:15248–57.
Gabellini D, Tupler R, Green MR. Transcriptional derepression as a cause of genetic diseases. Curr Opin Genet Dev. 2003;13:239–45.
Tiacci E, Grossmann V, Martelli MP, Kohlmann A, Haferlach T, Falini B. The corepressors BCOR and BCORL1: two novel players in acute myeloid leukemia. Haematologica. 2012;97:3–5.
Damm F, Chesnais V, Nagata Y, Yoshida K, Scourzic L, Okuno Y, et al. BCOR and BCORL1 mutations in myelodysplastic syndromes and related disorders. Blood. 2013;122:3169–77.
Bonnefont J, Tiberi L, van den Ameele J, Potier D, Gaber ZB, Lin X, et al. Cortical neurogenesis requires Bcl6-mediated transcriptional repression of multiple self-renewal-promoting extrinsic pathways. Neuron 2019;103:1096–108.e4.
Zhou D, Xue J, Gavrialov O, Haddad GG. Na+/H+ exchanger 1 deficiency alters gene expression in mouse brain. Physiol Genom. 2004;18:331–9.
Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–34.
Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9:213–24.
Karaca E, Li X, Lewicki J, Neofytou C, Guérout N, Barnabé-Heider F, et al. The corepressor CtBP2 is required for proper development of the mouse cerebral cortex. Mol Cell Neurosci. 2020;104:103481.
Mizuhara E, Minaki Y, Nakatani T, Kumai M, Inoue T, Muguruma K, et al. Purkinje cells originate from cerebellar ventricular zone progenitors positive for Neph3 and E-cadherin. Dev Biol. 2010;338:202–14.
Redies C, Neudert F, Lin J. Cadherins in cerebellar development: translation of embryonic patterning into mature functional compartmentalization. Cerebellum. 2011;10:393–408.
Acknowledgements
We would like to thank members and affiliates of the International Research Consortium for the Corpus Callosum and Cerebral Connectivity (IRC5, https://www.irc5.org) for their collaboration and input.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final paper and agree to be accountable for all aspects of the work. MG—data curation; writing—original draft. MM—data curation; writing—original draft. EA—data curation. KY—formal genetic analysis. EHS—data curation. KCP—data curation. EME—data curation. RHC—conceptualization. ZL—conceptualization. DL—conceptualization, formal genetic analysis. YMY—conceptualization, formal genetic analysis. TLS—conceptualization, writing—review and editing, supervision. LB—Conceptualization, writing—review and editing, supervision.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Consent for publication
Informed consent for publication was obtained from participants and their parents.
Ethics approval and consent to participate
The study was approved by the institutional review board [0075-17WOMC]. Informed consent to participate in the study was obtained from legal guardians.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gafner, M., Michelson, M., Argilli, E. et al. Major brain malformations: corpus callosum dysgenesis, agenesis of septum pellucidum and polymicrogyria in patients with BCORL1-related disorders. J Hum Genet 67, 95–101 (2022). https://doi.org/10.1038/s10038-021-00971-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-021-00971-5
This article is cited by
-
H2A monoubiquitination: insights from human genetics and animal models
Human Genetics (2023)