Abstract
Background:
Preterm very-low-birth-weight (VLBW) infants weighing <1.5 kg at birth develop anemia, often requiring multiple red blood cell transfusions (RBCTx). Because laboratory blood loss is a primary cause of anemia leading to RBCTx in VLBW infants, our purpose was to simulate the extent to which RBCTx can be reduced or eliminated by reducing laboratory blood loss in combination with pharmacodynamically optimized erythropoietin (Epo) treatment.
Methods:
Twenty-six VLBW ventilated infants receiving RBCTx were studied during the first month of life. RBCTx simulations were based on previously published RBCTx criteria and data-driven Epo pharmacodynamic optimization of literature-derived RBC life span and blood volume data corrected for phlebotomy loss.
Results:
Simulated pharmacodynamic optimization of Epo administration and reduction in phlebotomy by ≥55% predicted a complete elimination of RBCTx in 1.0–1.5 kg infants. In infants <1.0 kg with 100% reduction in simulated phlebotomy and optimized Epo administration, a 45% reduction in RBCTx was predicted. The mean blood volume drawn from all infants was 63 ml/kg: 33% required for analysis and 67% discarded.
Conclusion:
When reduced laboratory blood loss and optimized Epo treatment are combined, marked reductions in RBCTx in ventilated VLBW infants were predicted, particularly among those with birth weights >1.0 kg.
Similar content being viewed by others
Main
During the early weeks of life, anemia of prematurity develops in all surviving very-low-birth-weight (VLBW) preterm infants (birth weight (BW) < 1.5 kg). The duration and severity of anemia experienced by infants places them among the most highly transfused patient groups (1). This anemia is the result of prematurity itself, laboratory blood sampling, shortened red blood cell (RBC) life span, inadequate erythropoiesis, hemorrhage, and unidentified factors (2). These contributors all tend to occur more commonly among extremely-low-birth-weight (ELBW) preterm infants (BW < 1.0 kg) whose anemia and need for RBC transfusions (RBCTx) is particularly pronounced. Clinically significant anemia is treated with RBCTx. Because the number of RBC transfusions that critically ill premature infants receive is associated with increased mortality (3) and because RBCTx themselves are associated expense and complications (4), a reduction in the number of RBC VLBW infants receive is desirable.
Two important strategies to reduce RBCTx include: treatment with recombinant human erythropoietin (Epo) and reduction in laboratory phlebotomy blood loss. Although Epo treatment has been effective in reducing the number of RBCTx infants receive (5) and may have potentially beneficial neuroprotective properties (6), its administration in premature infants remains has been controversial because of its relative modest efficacy in previous Epo dosing schedules (5) and because of its prior erroneous association with retinopathy of prematurity (R. Ohls, personal communication). Reducing laboratory phlebotomy loss has been another promising strategy in decreasing RBCTx (7). Because the total blood volume removed from preterm infants for laboratory testing frequently exceeds their total blood volume at birth (2,8) and because laboratory phlebotomy loss has been directly correlated with the volume of RBCs transfused (2), reducing laboratory phlebotomy loss has considerable potential as an effective strategy for reducing RBCTx.
Although these two strategies for reducing RBCTxs in preterm infants have shown promise (8,9), application of a combination of these strategies has greater cumulative potential for reducing RBCTxs than does either of these alone. Thus, the purpose of this study was to determine the probability of success in which RBCTx can be eliminated in a significant proportion of preterm infants by optimizing Epo dosing in combination with a reduction in laboratory phlebotomy loss.
Results
Study Subjects
Of the 26 VLBW study subjects meeting study eligibility criteria, 18 were in the subgroup of ELBW infants and 8 were in the subgroup with birth weights between 1.0 and 1.5 kg. During the 30-d study, the latter subgroup received 1.75 ± 0.71 (mean ± SD) RBCTx per infant while the ELBW infants received 4.83 ± 1.72 RBCTx. Of the 2,656 laboratory blood samples drawn from all subjects, 97% were weighed before analysis. When adjusted for BW, 63.0 ± 32.5 ml/kg per infant was removed from the entire group. This corresponded to 30.5 ± 9.5 ml/kg for VLBW infants weighing from 1.0 to 1.5 kg, and 74.1 ± 25.3 ml/kg for ELBW infants (P < 0.05).
Analysis of Usage of Phlebotomized Blood
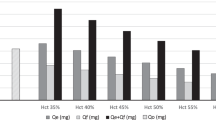
Analysis of the blood removed for laboratory testing over the first month of life demonstrated that 33% percent of the volume of blood required by test instruments was required for analysis. Of the remaining 67% of blood removed, 8% was unrecoverable in syringes and on gauze pads, bedding, band aides, etc. The remaining 59% was not used in the analysis. To characterize laboratory blood sampling, blood samples were categorized by the type of individual laboratory tests performed. Data for the entire study population was analyzed alone and on subgroups of VLBW infants weighing <1.0 kg and weighing from 1.0 to 1.5 kg ( Figure 1 ). In the analysis, blood sampled was expressed both as ml/kg birth weight ( Figure 1a ) and as percentage of the total blood volume removed ( Figure 1b ). Nine individually selected laboratory test categories provided information regarding the specific testing performed, e.g., hemoglobin (Hb), electrolytes, bilirubin, etc. The three individually selected test categories on the left of Figure 1 comprised 67% of the total blood volume drawn. For the individual “miscellaneous” category comprising ~20% of the total blood volume sampled, only 4.1% of the volume sampled was required by the instrument for analysis. This compares to the 33% required for all blood tests combined. When the two VLBW birth weight subgroups were compared, infants in the <1.0 kg BW subgroup had significantly greater blood volumes drawn relative to the 1.0–1.5 kg subgroup for all individually selected laboratory test categories (P < 0.05).
Most laboratory blood sampled in very-low-birth-weight preterm infants is lost or discarded. (a) Mean (±SD) total blood sampled during the first month of life by individually selected laboratory test categories (indicated in the legend at the bottom of the figure) grouped by birth weight (<1.0 kg: n = 18; 1.0–1.5 kg: n = 8; and <1.5 kg: n = 26) in ml per kg birth weight. (b) The same data are expressed as cumulative percentages of the individually selected test categories in the same birth weight groupings. The black bars represent the blood volume required for analysis by specific test instruments, and the white bars represent the remaining leftover blood. Individually selected laboratory test categories with the analytes included are as follows: (i) syringe pH/blood gas: arterial or venous pH and blood gases, electrolytes, glucose, lactic acid, total bilirubin, hemoglobin, methemoglobin, and ionized calcium; (ii) capillary radiometer 95: capillary pH and blood gases, electrolytes, glucose, lactic acid, total bilirubin, hemoglobin, methemoglobin, and ionized calcium; (iii) miscellaneous drugs and chemistries: miscellaneous drug levels and chemistries, e.g., creatinine, hormone levels, direct bilirubin, C-reactive protein; (iv) complete blood count; (v) capillary radiometer 55: hemoglobin, capillary pH, and blood gases, total bilirubin, and methemoglobin; (vi) blood culture; (vii) coagulation profile; (viii) capillary radiometer 35: hemoglobin, methemoglobin, total bilirubin, or glucose and lactic acid; and (ix) blood type and metabolic screen.
Simulated Hb Profiles With Phlebotomy Reduction and Optimized Epo Dosing
For the 26 preterm study infants, simulated Hb profiles during the first month of life were based on hypothetical reductions of laboratory phlebotomy loss in combination with simulated optimized Epo dosing. In Figure 2 , the simulated mean Hb profiles with 0, 50, and 100% reduction in phlebotomy were compared to the subject’s mean actual Hb concentration. As a result of the numerous RBCTx and phlebotomies performed, the actual mean Hb concentration profile of all study subjects demonstrated an erratic, generally horizontal pattern. When optimized Epo dosing was combined with a 100% reduction in laboratory phlebotomy, i.e., 0% phlebotomy loss, modeled Hb concentration was maintained at a comparable level to that actually observed for mean Hb concentration for all but the last few days of the study period.
Simulated erythropoietin (Epo) dosing with phlebotomy reduction predicts hemoglobin (Hb) concentrations similar to actual Hb concentrations over the first month of life. The erratic, flat horizontal solid line represents the actual mean Hb levels measured for all study subjects. The bottom smoothed dashed line is the simulated Hb concentration with optimized Epo administration and no reduction in laboratory phlebotomy loss. The second solid smooth line is the simulated Hb concentration with optimized erythropoietin (Epo) administration and a 50% reduction in laboratory phlebotomy loss. The top broken line is the simulated Hb concentration with both optimized Epo treatment and 100% reduction in laboratory phlebotomy loss.
Avoiding RBCTx With Optimal Epo Dosing and Phlebotomy Reduction
Using simulated Hb concentrations for individual study subjects, the model predicted the number of RBCTx administered for the two different RBCTx criteria applied. These two included the actually observed and the criteria applied in the premature infants in need of transfusion (PINT) study ( Figures 3 and 4 ). As expected, a higher percentage of VLBW infants in the subgroup with birth weights from 1.0 to 1.5 kg were predicted to avoid RBCTx compared to ELBW infants. With 100% reduction in phlebotomies without simulated Epo dosing, none of the <1.0 kg infants were predicted to avoid RBCTx completely (data not shown). A consistent finding in the simulations of both VLBW subgroups was that the number of infants predicted to avoid RBCTx showed an abrupt increase in RBCTx reduction with a 50–80% reduction in laboratory blood loss.
Percent of infants with birth weight from 1.0 to 1.5 kg predicted to completely avoid red blood cell transfusions (RBCTx). Shown for this subgroup are: top panel a: simulated result for phlebotomy reduction alone and bottom panel b: simulation result for phlebotomy reduction plus erythropoietin (Epo) administration (n = 8). The dashed line represents the premature infants in need of transfusion RBCTx criteria and the solid line represents the actual RBCTx practice applied to individual study subjects.
Percent of infants with birth weight <1.0 kg predicted to completely avoid red blood cell transfusions (RBCTx) with simulated phlebotomy reduction and erythropoietin (Epo) administration (n = 18). The dashed line represents simulations results according to the premature infants in need of transfusion RBCTx criteria while the solid line represents the simulation results from actual RBCTx practice.
Discussion
Using state-of-the-art pharmacokinetics and pharmacodynamics (PK/PD) simulation modeling, we were able to predict the number of RBCTx received by infants following concurrent optimization of Epo dosing and simulated reduction in laboratory blood loss. With optimized Epo dosing and elimination of all laboratory phlebotomy loss, modeling predicted that RBCTx would be eliminated in all infants with BW 1.0–1.5 kg and in 45% of infants with BW < 1.0 kg. Since the simulated therapeutic interventions in this study are hypothetical and therefore speculative, their feasibility and accuracy must be prospectively examined. In this regard, although optimized Epo dosing applied in this study can be examined immediately for its ability to increase Hb production, future technological improvement in laboratory instrumentation combined with nonpharmacological interventions to reduce laboratory blood loss will be required to significantly reduce laboratory blood loss (7).
Epo Administration
There have been numerous clinical trials in which Epo was administered to preterm infants with the goal of reducing or eliminating RBCTx (5). Although many of these studies were successful in reducing neonatal RBCTx, the routine use of Epo remains controversial because of its modest efficacy in reducing RBCTx (5) and because a retrospective meta-analysis that erroneously identified an association of Epo treatment begun in the first week of life with severe retinopathy of prematurity (R. Ohls, personal communication). It is important to note that when Epo is administered at high doses, it may have neuroprotective properties (6,10). These promising observations require additional prospective clinical studies before a potential neuroprotective role of Epo administration in preterm infants can be confirmed or denied.
Phlebotomy Reduction
The current study demonstrated that 67% of the blood taken from infants for analysis was discarded or lost on to surfaces while only 33% was required for laboratory analysis. Thus, requesting sample volumes closer to those needed for individual analyses would reduce neonatal blood loss. Another alternative for reducing laboratory blood loss is to assay diluted samples (11). Clinical justification for the excess volumes of blood drawn from neonates is that it permits sample reanalysis when necessary, e.g., if results are questionable because of instrument error, malfunction, or observing critical outlier values. Although such occurrences exist, their frequency is uncertain. Further discussion is needed regarding whether the magnitude of excess blood withdrawn in neonates is justified.
Postdelivery laboratory phlebotomy loss at the time critically ill infants are admitted to the neonatal intensive care unit is the most intensive period of phlebotomy loss. An effective approach to mitigate this loss is to perform initial laboratory tests on residual placental blood (12). Combining this approach with delayed cord clamping or milking (13) to increase the initial circulating blood volume could further reduce RBCTx and associated risks.
Clinical trials utilizing point-of-care analyzers and in-line monitors to decrease phlebotomy loss have also demonstrated reductions in RBCTx administered to preterm infants (14,15). Current blood monitors are capable of analyzing blood gases, electrolytes, and Hb ex vivo before returning the blood analyzed to the infant. We previously speculated that if the menu offered by blood monitors were expanded to include glucose, bilirubin, blood urea nitrogen, and creatinine, 80% reduction in phlebotomy is achievable (15).
Criteria Applied in Administering RBCTx
The optimal RBCTx trigger criteria for VLBW infants is been a long-standing subject of controversy not addressed by the present data. Nonetheless, in practice when to transfuse is most commonly based on the infant’s Hb concentration, clinical status and/or postnatal age (15,16,17). In the current study, PK/PD simulation modeling was performed using the RBCTx criteria applied in the PINT study for infants with respiratory illness (17). In this analysis, a significant 50% reduction in phlebotomized blood loss with Epo administration was predicted as sufficient to maintain average Hb concentrations close to the actual observed mean Hb concentration. As better evidence-based RBCTx criteria are developed, simulations performed in this study can be readily modified to model predictions of RBCTx data for VLBW infants or other patient populations in which Epo therapy and blood loss are important.
Combination Therapy for Avoiding RBCTx
The current study is consistent with the study of Rabe et al. (9) that showed that a combination of interventions can reduce neonatal RBCTx to a greater extent than individual interventions alone. As expected, we observed that a greater proportion of 1.0–1.5 kg infants were predicted to avoid RBCTx compared to infants with BW < 1.0 kg. When the simulations were performed using the PINT trial’s restrictive RBCTx criteria, a greater number of infants were predicted to avoid RBCTx than the number who actually received RBCTx. Thus, transfusion avoidance occurs due to increased erythropoiesis accompanying the simulated Epo dosing relative to the natural, nonstimulated erythropoiesis. When the percent phlebotomy reduction was increased from 50 to 80%, there was a substantial increase in the number of infants predicted to avoid RBCTx for both BW subgroups ( Figures 3 and 4 ).
Benefits in Avoiding RBCTx in Preterm Infants
Since Epo administration (5) and utilization of restrictive RBCTx criteria (18) remain controversial therapeutic interventions for reducing RBCTx among VLBW infants, evidence is needed showing that the avoidance of RBCTx leads to better, more cost-effective outcomes. RBCTx cost is between $522 and $1,183 per unit (19). In addition to these costs, complications associated with RBCTx include infection, fluid overload, electrolyte imbalance, exposure to plasticizers, lead, and other toxins (5), and possibly necrotizing enterocolitis (20).
Limitations of Modeling Simulations
There were several limitations in predicting the number of RBCTx that infants were anticipated to receive. Predictions were based on estimates of blood volume, transfused RBC life span, and PD parameters. Blood volumes were estimated from the pre- and posttransfusion Hb level when analyzed by the dilution principle (21) and assuming 100% survival of transfused allogeneic donor RBCs (22). The mean blood volume observed, i.e., 93.2 ± 24.9 ml/kg, was within the range reported for preterm infants (21,23,24). The life span of adult donor transfused RBCs is commonly referenced as 120 d based on healthy adults in steady-state erythropoiesis (25). It is possible that transfused RBCs have a shorter life span in VLBW infants (26).
The PK/PD model used in the present simulations has several limitations. These include the lack of feedback for the endogenous Epo level and the inability to consider the Epo receptor state. With regard to the first limitation, the administration of optimized Epo is likely to decrease the endogenous Epo production (27). Nonetheless, since Epo concentrations used in the simulations are far higher than endogenous Epo concentrations, the effect is negligible. Regarding the second limitation, the pharmacodynamic model applied does not take into account dynamic changes in the Epo receptor state known to influence Epo PD (28). Although reliable measures of Epo receptors are not currently available for preterm infants, the simulation model applied is easily modifiable to accommodate new information.
In summary, simulations performed in this study suggest that future clinical trials that simultaneously apply phlebotomy reduction measures and optimized Epo administration can significantly reduce, and in some cases eliminate, the number of RBCTx administered to preterm VLBW infants when standardized RBCTx criteria in current clinical practice are applied.
Methods
Subjects
The study was approved by the University of Iowa Committee on Research on Human Subjects. Informed written parental consent was obtained. Study subjects included have been reported in previous publications with different primary objectives (8,29,30).
Subjects eligible for enrollment included the offspring of women presenting in labor at <29 wk gestation whose infants delivered at <29 wk gestation and were intubated in the first day of life. Infants excluded were those presenting with hematological disease (except for anemia of prematurity), those receiving RBCTx prior to enrollment, or those receiving erythropoiesis-stimulating agents. A total of 162 mothers or infants met study eligibility criteria. Of those eligible for enrollment, 119 were not approached because: (i) they had been approached for another study (n = 39); (ii) of the significant additional workload imposed on clinical laboratory personnel allowing them to include only two research subjects for study at a time (n = 62); (iii) prior blood transfusion before consent (n = 13); and (iv) lack of staff availability (n = 5).
A total of 43 families were approached: 11 before delivery and 32 after delivery. Consent was obtained from 33 families while 10 families refused. Women who consented antenatally but who delivered at >29 wk became ineligible (n = 6). Twenty-seven infants were enrolled and studied for approximately the first month of life (31.6 ± 2.2 d). The one infant who did not receive any RBCTx during the first month of life was omitted from analysis.
VLBW study subjects were divided into two subgroups: those with BW from 1.0 to 1.5 kg, and those with BW < 1.0 kg. This was done because it is well established that infants <1.5 kg receive increasing numbers of RBCTx with decreasing BW (2).
Study Procedures
Demographic, laboratory, and RBCTx data were obtained from the electronic medical record. Data included the specific laboratory test performed, if sampling was done using a syringe through a central arterial or venous catheter or by peripheral capillary heelstick, the volume of blood required by the test instrument, and the blood volume requested by laboratory personnel. Weights of 97% of all 2,656 individual laboratory blood samples drawn (138 ± 21 tests per infant) were determined to the nearest 0.1 mg prior to analysis (29). Following clinical analysis, leftover blood was centrifuged and the plasma or serum was frozen at −70 °C for later analysis. Leftover anticoagulated blood samples less than 3 d old were analyzed for hematological parameters using the Sysmex XE-2100 hematology analyzer (Sysmex, Kobe, Japan). Plasma Epo levels required for Epo optimization were analyzed using a radioimmunoassay (28).
Overview of the Simulation Modeling Applied
The computer simulations were designed to predict the number of RBCTx administered to preterm infants in combination with model optimized exogenous Epo dosing (30) in combination with concurrent reduction in laboratory phlebotomy loss (8). Predictions regarding the number of RBCTx administered were based on Hb cutoff levels for each of two different RBCTx criteria: (i) the actual Hb levels at which RBCTx were administered and (ii) the modeled Hb levels when applying restrictive PINT study criteria for infants receiving respiratory support (17). The PINT study’s “restrictive” RBCTx criteria were selected because they were employed in the largest randomized RBCTx trial in preterm infants. Modeled Hb data were determined from simulations utilizing optimized Epo dosing and modeled phlebotomy reductions as described below. Also required for the modeling predictions of reductions in RBCTx were Epo PK/PD data and blood volume and RBC life span data from the literature.
Epo Pharmacokinetic and Pharmacodynamic Optimization
Simulation of optimized Epo dosing requires both Epo PK and PD information. The elimination of Epo from the circulation occurs via a saturable, receptor-mediated pathway (31) in which the PK disposition model applied is given by:

where Epo is the exogenous simulated plasma Epo concentration at time t; P1 and P2 are disposition parameters, P1 is the maximum rate at which the exogenous Epo concentration declines following intravenous administration (U/ml․h), P2 is the plasma Epo concentration at which Epo is eliminated at half its maximum rate (U/ml). The parameters in Equation 1 have been determined in the same group of preterm VLBW infants in a prior study (30). All Epo doses were simulated as an intravenous bolus with a bioavailability of 1.
The Epo optimization used the same Emax model we previously described (29,30):

where E(t) is the Hb production rate at time t; tlag is the lag time between the Epo stimulation of Epo receptors and the appearance of newly synthesized reticulocytes, i.e., new Hb appearing in the circulation; EC50 is the plasma concentration of Epo resulting in half of the maximum Hb production rate; Emax is the maximum achievable Hb production rate. The Emax parameter of individual study infants was determined previously (30).
Phlebotomy Reduction Applied in the Model
Phlebotomy for laboratory testing results in a decline in Hb levels due to removal of both endogenous and transfused donor RBCs. The mathematical model applied to simulate infant Hb concentrations following different degrees of phlebotomy reduction has been previously reported by our group (8). To determine the Hb produced from simulated Epo doses while variably decreasing laboratory phlebotomy loss, we expanded our previous model as follows:

where Hb(t)EPO is the amount of Hb produced from Epo at time t (determined by Equation 2); Hb(t)EPO,PHLE is the amount of Hb produced from Epo in the presence of phlebotomies at time t; k is the number of phlebotomies which occurred before the current time; j is the given phlebotomy number; Fj is the fraction of the total blood volume removed in phlebotomy j. Equation 3 contains no correction factor for RBC senescence because the assumed 66-d life span of the newly produced cells is longer than the study period, i.e., 30 d (29).
Epo Dosing Schedule and RBCTx Simulation
The same Epo dosing schedule used in our prior optimal Epo modeling simulation study of preterm infants was applied in the current study, i.e., 12 individual intravenous 600 U/kg Epo doses optimized for timing. Optimized Epo dosing based on endogenous Epo PK/PD data showed potential for completely eliminating RBCTx in infants using Epo doses previously reported (32).
Following combined, concurrent simulation of both optimized Epo dosing and laboratory phlebotomy reduction, Hb profiles of individual infants were analyzed according to the two RBCTx criteria described above. When an individual infant’s simulated Hb concentration fell below one of these two specific RBCTx criteria, it was counted as a simulated RBCTx. All clinically simulated RBCTx were assumed to be 15 ml/kg of packed RBCs with an assumed Hct of 83.1% (8). Simulations were programmed in FORTRAN with graphical output performed using WINFUNFIT (33) or Microsoft Excel (Microsoft Redmond, WA).
Statement of Financial Support
This study was supported by National Institutes of Health (NIH, Bethesda, MD) grant P01 HL046925 and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH, through grant UL1RR024979. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
The authors have nothing to disclose.
References
Levy GJ, Strauss RG, Hume H, et al. National survey of neonatal transfusion practices: I. Red blood cell therapy. Pediatrics 1993;91:523–9.
Widness JA . Pathophysiology of Anemia During the Neonatal Period, Including Anemia of Prematurity. Neoreviews 2008;9:e520.
dos Santos AM, Guinsburg R, de Almeida MF, et al.; Brazilian Network on Neonatal Research. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr 2011;159:371–376.e1–3.
Galel SA, Fontaine MJ . Hazards of neonatal blood transfusion. Neoreviews 2006;7:e69–e74.
Ohlsson A, Aher SM . Early erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database Syst Rev 2012;9:CD004863.
McCoy TE, Conrad AL, Richman LC, Lindgren SD, Nopoulos PC, Bell EF . Neurocognitive profiles of preterm infants randomly assigned to lower or higher hematocrit thresholds for transfusion. Child Neuropsychol 2011:1–21.
Carroll PD, Widness JA . Nonpharmacological, blood conservation techniques for preventing neonatal anemia–effective and promising strategies for reducing transfusion. Semin Perinatol 2012;36:232–43.
Rosebraugh MR, Widness JA, Nalbant D, Veng-Pedersen P . A mathematical modeling approach to quantify the role of phlebotomy losses and need for transfusions in neonatal anemia. Transfusion 2013;53:1353–60.
Rabe H, Alvarez JR, Lawn C, Seddon P, Amess PN . A management guideline to reduce the frequency of blood transfusion in very-low-birth-weight infants. Am J Perinatol 2009;26:179–83.
Juul S . Erythropoietin in anemia of prematurity. J Matern Fetal Neonatal Med 2012;25:Suppl 5:80–4.
Freise KJ, Schmidt RL, Gingerich EL, Veng-Pedersen P, Widness JA . The effect of anticoagulant, storage temperature and dilution on cord blood hematology parameters over time. Int J Lab Hematol 2009;31:496–504.
Carroll PD, Nankervis CA, Iams J, Kelleher K . Umbilical cord blood as a replacement source for admission complete blood count in premature infants. J Perinatol 2012;32:97–102.
Rabe H, Jewison A, Alvarez RF, et al.; Brighton Perinatal Study Group. Milking compared with delayed cord clamping to increase placental transfusion in preterm neonates: a randomized controlled trial. Obstet Gynecol 2011;117(2 Pt 1):205–11.
Madan A, Kumar R, Adams MM, Benitz WE, Geaghan SM, Widness JA . Reduction in red blood cell transfusions using a bedside analyzer in extremely low birth weight infants. J Perinatol 2005;25:21–5.
Widness JA, Madan A, Grindeanu LA, Zimmerman MB, Wong DK, Stevenson DK . Reduction in red blood cell transfusions among preterm infants: results of a randomized trial with an in-line blood gas and chemistry monitor. Pediatrics 2005;115:1299–306.
Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics 2005;115:1685–91.
Kirpalani H, Whyte RK, Andersen C, et al. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr 2006;149:301–7.
Bell EF . When to transfuse preterm babies. Arch Dis Child Fetal Neonatal Ed 2008;93:F469–73.
Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR . Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion 2010;50:753–65.
La Gamma EF, Blau J . Transfusion-related acute gut injury: feeding, flora, flow, and barrier defense. Semin Perinatol 2012;36:294–305.
Rawlings JS, Pettett G, Wiswell TE, Clapper J . Estimated blood volumes in polycythemic neonates as a function of birth weight. J Pediatr 1982;101:594–9.
Strauss RG, Mock DM, Widness JA, Johnson K, Cress G, Schmidt RL . Posttransfusion 24-hour recovery and subsequent survival of allogeneic red blood cells in the bloodstream of newborn infants. Transfusion 2004;44:871–6.
Schulman I, Smith CH, Stern GS . Studies on the anemia of prematurity. AMA Am J Dis Child 1954;88:567–95.
Usher R, Lind J . Blood volume of the newborn premature infant. Acta Paediatr Scand 1965;54:419–31.
Steinberg MH, Benz EJ, Jr., Adewoye HA, Ebert B . Pathobiology of the human erythrocyte and its hemoglobins. In: Hoffman R, Benz EJ, Shattil SJ, Furie B, Cohen HJ, Silberstein LE, McGlave P, eds. Hematology: Basic Principles and Applications. New York, NY: Elsevier, 2005: 442–54.
Landaw SA . Factors that accelerate or retard red blood cell senescence. Blood Cells 1988;14:47–67.
Woo S, Krzyzanski W, Jusko WJ . Target-mediated pharmacokinetic and pharmacodynamic model of recombinant human erythropoietin (rHuEPO). J Pharmacokinet Pharmacodyn 2007;34:849–68.
Rosebraugh M, Widness JA, Veng-Pedersen P . Receptor-based dosing optimization of erythropoietin in juvenile sheep after phlebotomy. Drug Metab Dispos 2011;39:1214–20.
Freise KJ, Widness JA, Veng-Pedersen P . Erythropoietic response to endogenous erythropoietin in premature very low birth weight infants. J Pharmacol Exp Ther 2010;332:229–37.
Rosebraugh MR, Widness JA, Veng-Pedersen P . Multidose optimization simulation of erythropoietin treatment in preterm infants. Pediatr Res 2012;71(4 Pt 1):332–7.
Sawyer ST, Krantz SB, Goldwasser E . Binding and receptor-mediated endocytosis of erythropoietin in Friend virus-infected erythroid cells. J Biol Chem 1987;262:5554–62.
Juul SE, McPherson RJ, Bauer LA, Ledbetter KJ, Gleason CA, Mayock DE . A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 2008;122:383–91.
Veng-Pedersen P . Curve fitting and modeling in pharmacokinetics and some practical experiences with NONLIN and a new program FUNFIT. J Pharmacokinet Biopharm 1977;5:513–31.
Acknowledgements
The rabbit erythropoietin antiserum used in the erythropoietin radioimmunoassay was a gift from Gisela K. Clemens. We appreciate the outstanding contributions of the clinical laboratory staff led by Mitchell J. Owen with oversight from Matthew D. Krasowski. We also appreciate the substantial contributions from our laboratory research team (Robert L. Schmidt, Earl L. Gingerich, and Jessica A. Goehring) and our nursing research team (Gretchen A. Cress, Karen J. Johnson, Nancy E. Krutzfield, Sara K.B. Scott, and Ruthann Schrock). Manuscript review and critique by Patrick Carroll, was helpful and insightful. Mark A. Hart provided expert editorial and secretarial help. Finally, we are grateful to Sysmex America for the loan of the Sysmex XE-2100 hematology automated analyzer.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosebraugh, M., Widness, J., Nalbant, D. et al. Pharmacodynamically optimized erythropoietin treatment combined with phlebotomy reduction predicted to eliminate blood transfusions in selected preterm infants. Pediatr Res 75, 336–342 (2014). https://doi.org/10.1038/pr.2013.213
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2013.213
This article is cited by
-
Effects of blood sampling stewardship and erythropoietin administration in extremely low birth weight infants—a quality improvement non-controlled before-and-after retrospective study
European Journal of Pediatrics (2021)
-
Erythropoietin prevents necrotizing enterocolitis in very preterm infants: a randomized controlled trial
Journal of Translational Medicine (2020)
-
Association between anemia and bronchopulmonary dysplasia in preterm infants
Scientific Reports (2016)
-
Why do four NICUs using identical RBC transfusion guidelines have different gestational age-adjusted RBC transfusion rates?
Journal of Perinatology (2015)