Abstract
Introduction:
There is little information regarding the comparative hemodynamic effects of adding milrinone or levosimendan to dopamine infusion in hypoxia-reoxygenated (H-R) newborns.
Results:
Severely hypoxic piglets had cardiogenic shock with depressed cardiac index (CI) and mean arterial pressure (MAP). The hemodynamics deteriorated gradually after initial recovery upon reoxygenation. Heart rate and CI improved with milrinone (D+M) and levosimendan (D+L) administration (P < 0.05 vs. control). Both regimens improved carotid arterial flow and carotid vascular resistance; D+M additionally improved superior mesentric arterial flow (all P < 0.05 vs. control). No effect was found on renal arterial flow or elevated lactate state with either regimen. D+M piglets also had a lower myocardial oxidized/reduced glutathione ratio (P < 0.05 vs. control).
Discussion:
In conclusion, adding milrinone or levosimendan to dopamine similarly improved systemic hemodynamics in H-R newborn piglets. Milrinone also improved mesenteric perfusion and attenuated myocardial oxidative stress.
Methods:
Twenty-eight piglets (1–4 d, 1.5–2.5 kg) were instrumented for continuous monitoring of systemic MAP and pulmonary arterial pressure (PAP), CI, and carotid, superior mesenteric, and renal arterial flows. Piglets were randomized with blinding to sham-operated, H-R control (saline), and H-R dopamine (10 μg/kg/min) with D+M or D+L groups. H-R piglets underwent H-R followed by 2 h of drug infusion after reoxygenation. Tissue was collected for biochemical/oxidative stress testing and histological analysis.
Similar content being viewed by others
Main
Asphyxia of the neonate is often complicated by cardiovascular dysfunction (1,2). Neonates may exhibit hypotension, pulmonary hypertension, and shock with end-organ dysfunction (3,4). Moreover, following resuscitation, the newborn myocardium may be stunned secondary to reperfusion injury (5). Catecholamines such as dopamine, epinephrine, and dobutamine are used to support the cardiovascular status of these neonates. Dopamine is often used as the initial vasoactive agent infused following resuscitation (6). High doses of dopamine are typically avoided in neonatal shock given concerns of severe vasoconstriction and tachycardia (7). It is common to add an inotrope in combination with dopamine infusion.
The commonly used catecholamines have α- and β-adrenergic effects. As the aforementioned resuscitation strategy stated, although it is a common practice to add adrenergic catecholamine to critically sick neonates treated with dopamine infusion, the drugs may cause further stress to the compromised myocardium following asphyxia and reoxygenation. To improve contractility and perfusion, some clinicians may add noncatecholamine agents. Milrinone is a phosphodiesterase III inhibitor with inotropic, lusitropic, and vasodilatory properties (6). Recently, we demonstrated that the infusion of milrinone in an established swine model of neonatal hypoxia-reoxygenation (H-R) increased mesenteric perfusion in a dose-dependent fashion (8,9). Levosimendan is a calcium-sensitizing agent that is still novel to neonatal medicine. Levosimendan functions to improve contractility and reduce afterload via binding to troponin C and opening of adenosine triphosphate-dependent K+-channels in vascular smooth muscle, respectively (10,11). Regional hemodynamic effects of levosimendan have not yet been reported.
Inotropic medications are frequently used in the setting of neonatal shock from asphyxia, albeit, without strong evidence for their use (4), nor their circulatory effects in regional vasculature. Although milrinone and levosimendan are novel second-line inotropes used to provide cardiovascular support with the potential reduction in afterload, there is no study available to compare the systemic and regional hemodynamic effects of these two agents as a second-line drug in hypoxic-reoxygenated newborn subjects, including a direct comparison of combination treatment strategies during dopamine administration. To make such a comparison, we used an animal model of neonatal H-R to compare the effects on hemodynamics of adding milrinone or levosimendan to a background infusion of dopamine. Additional outcomes assessed include the effects on oxygen metabolism, oxidative stress, and its related histologic tissue injury. We hypothesized that the addition of milrinone to dopamine would have beneficial effects on carotid, mesenteric, and renal perfusion as compared with the addition of levosimendan to dopamine in neonatal H-R.
Results
Of 28 piglets instrumented, 24 were analyzed following exclusions for complications related to surgery (one piglet) and hypoxia (three piglets). Animals were age 2.2 ± 0.2 d and weighed 1.9 ± 0.1 kg. Following stabilization, hemodynamic and metabolic parameters were not different across experimental groups.
Hypoxia and Reoxygenation
After 2 h of hypoxia (arterial partial pressure of oxygen 38 ± 2 mm Hg, Table 1 ), H-R piglets demonstrated systemic hypotension, pulmonary hypertension, and decreased stroke volume and cardiac index (CI) (all P < 0.05 vs. sham, Table 2 ). Corresponding reductions were found in systemic oxygen delivery and consumption (P < 0.05 vs. sham, data not shown). The perfusion of carotid, mesenteric, and renal circulation was reduced to various degrees compared with that of sham. Severe metabolic acidosis with associated hyperlactatemia was present in all H-R piglets (both P < 0.05 vs. sham, Table 1 ).
After 2 h of reoxygenation, H-R piglets had mean arterial pressure (MAP) of 46 ± 2 mm Hg ( Table 2 ). Stroke volume was depressed (58 ± 3% of normoxia baseline; H-R dopamine with milrinone (D+M) and H-R dopamine with levosimendan (D+L), P < 0.05 vs. sham), although no differences were found in CI, systemic oxygen delivery, or oxygen consumption relative to sham. Carotid perfusion was reduced in H-R piglets (60 ± 4% of normoxia baseline; P < 0.05 vs. sham), whereas mesenteric and renal perfusion (84 ± 8% and 97 ± 11% of normoxia baselines, respectively) were not significantly different from sham. Metabolic acidosis and hyperlactatemia persisted following reoxygenation (both P < 0.05 vs. sham, Table 1 ). Plasma troponin levels were also elevated in all H-R piglets following reoxygenation (P < 0.05 vs. sham, Table 1 ).
Systemic and Pulmonary Hemodynamic Responses
D+M and D+L piglets had increased heart rates during drug infusion (P < 0.05 vs. control, Figure 1a ). D+M piglets had modestly higher stroke volume over the course of infusion (P = 0.075 vs. control, Figure 1b ). The combined effect offered an increase in CI for both D+M and D+L (P < 0.05 vs. control, Figure 1c ), but MAP did not change ( Table 2 ). No disparate effect on pulmonary arterial pressure (PAP) was found either ( Table 2 ), although pulmonary vascular resistance decreased with medication infusion in both groups (P < 0.05 vs. control). Systemic vascular resistance was reduced (P < 0.05 vs. control). There were improvements in systemic oxygen delivery with D+M and D+L treatment (P < 0.05 vs. control), whereas oxygen consumption was not altered ( Figure 2 ).
Changes in (a) heart rate (HR) (beats/minute, BPM), (b) stroke volume index (SVI) and (c) cardiac index (CI) during infusion of placebo control (white bar), dopamine with milrinone (black bar), and dopamine with levosimendan (gray bar). Stroke volume index and cardiac index are expressed as mean percentage change over duration of infusion from predrug baseline. *P < 0.05 vs. control (two-way repeated-measures ANOVA). **P = 0.075 vs. control (two-way repeated-measures ANOVA).
Changes in (a) systemic oxygen delivery (syst DO2) and (b) consumption (syst VO2) during infusion of placebo control (white bar), dopamine with milrinone (black bar), and dopamine with levosimendan (gray bar). Data are expressed as mean percentage change over duration of infusion from predrug baseline. *P < 0.05 vs. control (two-way repeated-measures ANOVA).
Regional Hemodynamic Responses
Treatment with D+M and D+L improved carotid perfusion (increased carotid artery flow index and carotid oxygen delivery) with reduced carotid vascular resistance (all P < 0.05 vs. control, Figure 3 ). D+M but not D+L piglets had increases in superior mesenteric artery flow index and mesenteric oxygen delivery (both P < 0.05 vs. control) with no effect on vascular resistance ( Figure 3 ). There were no significant effects in renal perfusion.
Changes in carotid and mesenteric (a) flow, (b) oxygen delivery, and (c) vascular resistance during infusion of placebo control (white bar), dopamine with milrinone (black bar), and dopamine with levosimendan (gray bar). Data are expressed as mean percentage change over duration of infusion from predrug baseline. *P < 0.05 vs. control (two-way repeated-measures ANOVA).
Biochemical Effects and Histopathology
The myocardium of D+M piglets had a lower oxidized tissue glutathione (GSSG)/tissue glutathione (GSH) ratio than that of controls (P < 0.05, Figure 4 ). There were no differences in small intestine GSSG/GSH ratio or tissue malonyldialdehyde (data not shown). All H-R groups had plasma lactate levels that were not different from those of sham (P > 0.05), showing that at the doses studied, the treatments have no detrimental effects on tissue oxygenation and metabolism. H-R piglets also had similar pH, troponin, left ventricle and small intestinal lactate, and degree of histologic ischemia-reperfusion injury (data not shown).
Effect of infusion of placebo control (white bar), dopamine with milrinone (black bar), and dopamine with levosimendan (gray bar) on left-ventricular myocardial oxidative stress (GSSG/GSH ratio). A lower ratio indicates less oxidative stress. *P < 0.05 vs. control (one-way ANOVA). GSH, glutathione; GSSG, oxidized GSH.
Discussion
Asphyxiated neonates may present with myocardial depression and reduced tissue perfusion following resuscitation. Few studies are available in the literature examining the potential benefits of vasoactive medications in neonates, particularly following asphyxia (4,12). Further, the hemodynamic effects of combination therapy have not been systemically studied. We demonstrated that the addition of milrinone or levosimendan to moderate-dose dopamine infusion in H-R piglets improves systemic circulation with increases in CI, oxygen delivery, and carotid perfusion. Of note, systemic oxygen consumption was not affected despite of improvements in systemic oxygen delivery with both treatments. Milrinone, in particular, also improves mesenteric perfusion and attenuates oxidative stress in the newborn piglet myocardium.
The majority of literature on neonatal milrinone use focuses on the treatment of the low cardiac output state following bypass surgery and pulmonary hypertension (13,14,15,16), showing improvement in heart rate and CI. Reduction of vascular resistance and afterload also contribute to circulatory improvements with milrinone (17). Similar findings were recognized in a recent animal model where the increase in afterload while on dopamine following bypass surgery was prevented through the administration of milrinone or levosimendan, thereby preserving cardiac output (15). In patients with acute heart failure, levosimendan administration improved ejection fraction and abated the need for other inotropes (18,19). Nevertheless, the majority of evidence regarding levosimendan use in the neonate population is limited to case reports or series (18,19,20,21).
Previously, we showed the dose response of milrinone and levosimendan on systemic hemodynamics in H-R piglets (9,22,23). This study revealed similar findings on systemic circulation with chronotropic effects and improved cardiac output with both treatment regimens. It is unclear whether milrinone exerts inotropic effects during the first few days after delivery in immature animals (24) or the human neonate. In addition, vascular resistance was decreased through the addition of both milrinone and levosimendan, thereby reducing myocardial wall stress and potentially limiting any effect on myocardial oxygen consumption. Indeed, no differences were appreciated in myocardial lactate levels or in systemic oxygen consumption. Not found in our previous study of milrinone administered alone (9), D+M piglets’ myocardium exhibited less oxidative stress, as shown by the GSSG/GSH ratio, which reflects the intracellular redox status. Although the better preserved myocardial GSSG/GSH ratio in D+M piglets may indicate reduced reperfusion injury and presumably, preserved contractility, redox signaling is particularly relevant to activation of different gene pathways such as nuclear factor κ-B, the signal transducers, and activators of transcription protein and mitogen-activated protein kinases with chronic sequelae. Although malondialdehyde levels were similar across groups, the levels may reflect an acute oxidation of lipids, with controversial implications. Plasma troponin also was not different among H-R groups. This may be due to the temporal delay in the detection of elevated troponin levels. Indeed, troponin values increased only following the reoxygenation period. As such, longer duration of data collection would be necessary to determine if milrinone addition protects against myocardial injury during H-R.
Both milrinone and levosimendan have been described in the treatment of neonates with pulmonary hypertension (15,16,21,24,25,26) because of the potential benefit in unloading the right ventricle. We observed a reduction in pulmonary vascular resistance with the infusion of both regimens. Despite this and the reduction in right-heart afterload, no significant effect on PAP was observed. Although our piglets exhibited significant pulmonary hypertension during hypoxia and early reoxygenation, these increases were attenuated prior to infusion of medications. The latter may preclude the observation of pulmonary vascular effect of medications.
To our knowledge, none of the clinical studies of milrinone and levosimendan use have examined the regional effects of these agents. Administered alone in H-R newborn piglets, milrinone benefits the mesenteric and carotid circulation (8,9,22). Levosimendan administered as the sole agent in a similar animal model, on the other hand, has no effect on regional circulation (23). Both agents studied improved carotid perfusion when used in combination with dopamine. Although a direct correlation between carotid and cerebral blood flows has been demonstrated in animal studies (27), cautious interpretation of carotid hemodynamics is required because the common carotid artery supplies both cerebral and extracranial tissues. Further modest decreases in regional vascular resistance ( Figure 3c ) may partly explain the increases in regional blood flows. Nevertheless, the exact mechanism behind circulatory improvements observed with combination treatment requires further investigation. Similar to monotherapy studies, addition of milrinone to dopamine also significantly improved mesenteric perfusion. The small sample size may have precluded us from demonstrating significant but modest differences in mesenteric vascular resistance and perfusion between D+L and control piglets. The lack of an effect of the treatments on renal perfusion is probably explained by the fact that renal perfusion recovered by 2 h after the hypoxia challenge in the all H-R piglets before treatment started.
Hypercapnia often accompanies asphyxia and may affect hemodynamic recovery (28), whereas anesthetic agents may affect newborn swine hemodynamics and organ function. Nonetheless, our study model approximates the clinical scenario of neonatal asphyxia resulting in cardiogenic shock and tissue hypoperfusion after H-R. The study was designed before the 2010 International Liaison Committee on Resuscitation guidelines were published, thus 100% oxygen was applied for 30 min in the resuscitation, which can be devastating, especially in the human subject. The transition from fetal to neonatal circulation during the perinatal period may also affect the application of our findings in asphyxiated neonates (29). Despite all the recognizable limitations of our animal model, both piglet and human neonates are similar in size, cardiovascular function, and pathophysiological responses to H-R (30). Nonetheless, interspecies differences will impact the clinical applicability of our findings. There are no data yet suggesting that levosimendan or milrinone should be used in asphyxiated neonates in the clinical practice outside controlled clinical trials. Thus, clinical trials in human neonates are needed using novel technologies for continuous monitoring of systemic and regional hemodynamic and oxygen transport.
Levosimendan appears to be a reasonable agent to add to moderate-dose dopamine in asphyxiated neonates suffering shock. Potential benefits to systemic circulation were expressed without detrimental effects on systemic oxygen metabolism. Addition of milrinone, however, provides similar potential beneficial hemodynamic effects but also improves mesenteric perfusion with a possible benefit to the myocardium with less oxidative stress. Further studies are needed if the improvement in cardiac indexes will be more substantial after prolonged treatment and evidenced in clinical practice.
Methods
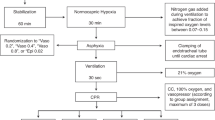
With approval of the Animal Care and Use Committee: Health Sciences (University of Alberta) and following the 2000 Canadian Council on Animal Care guidelines, mixed-breed 1- to 4-d-old piglets, weighing 1.5–2.5 kg, were obtained from the university swine research farm for experimentation. The experiment has been previously described (8,9). Briefly, animals were anesthetized initially using isoflurane, which was substituted with intravenous midazolam and fentanyl. Piglets were ventilated mechanically at pressures of 20/4 cm H2O and rates of 18–20 breaths/min (Sechrist Infant Ventilator Model IV-100, Sechrist Industries, Anaheim, CA) with fractionated inspired oxygen concentrations (FiO2) of 0.21–0.25. Oxygen saturation was monitored and maintained at 88–100% using pulse oximetry (Nellcor, Hayward, CA). Piglets were ventilated to a partial arterial pressure of carbon dioxide of 35–45 mm Hg throughout the protocol. Temperature was maintained from 38.5–40 °C with a heating underpad and overhead warmer.
The right femoral vein and artery were cannulated with 5-Fr dual-lumen and single-lumen catheters (Sherwood Medical, St. Louis, MO), respectively, and positioned for fluid and medication administration and central venous pressure and MAP monitoring, respectively. Flow probes (Transonic Systems, Ithaca, NY) were placed at the following positions: (i) left common carotid artery (2SS), (ii) superior mesenteric artery (3SB), (iii) left renal artery (2SB), and (iv) main pulmonary artery (6SB, as a surrogate of cardiac output with the ligation of ductus arteriosus). The main pulmonary artery was cannulated with a 20-gauge angiocatheter (Insyte-W, Becton Dickinson Infusion Therapy Systems, Sandy, UT) for PAP monitoring. Flow probe measurements were digitized (Data Translation, ON, Canada) and recorded with Asyst programming software on a personal computer. Following meticulous anesthesia and careful surgery, a period of stabilization (45–60 min) was allowed prior to commencing the H-R protocol.
H-R Protocol
After stabilization, block randomization was used to assign piglets to a surgical sham group or one of three H-R groups. Sham-operated piglets (sham, n = 6) were maintained at FiO2 of 0.21–0.25 throughout the experiment (6 h). H-R piglets underwent normocapnic alveolar hypoxia (FiO2 of 0.10–0.15 for 2 h) with arterial partial pressure of oxygen of 20–40 mm Hg to produce cardiac dysfunction and hypotension as previously described (9). Piglets were then resuscitated with 100% oxygen for 30 min and a 10 ml/kg bolus of lactated Ringer’s solution. FiO2 was then kept at 0.21–0.25 (3.5 h) to maintain normoxia (arterial oxygen saturation of 88–95%) during reoxygenation.
At 2 h of reoxygenation, H-R piglets received blinded infusions of either placebo (0.9% saline solution, control, n = 6) or study drug at a constant rate. Drug study groups received dopamine (10 μg/kg/min; Baxter, Toronto, ON, Canada) combined with either milrinone (50 μg/kg bolus then 0.5 μg/kg/min infusion; Apotex, Toronto, ON, Canada; n = 6, D+M) or levosimendan (24 μg/kg bolus then 0.2 μg/kg/min infusion; Abbott Laboratories S.A., Madrid, Spain; n = 6, D+L). Drug doses were determined following clinical practice and past dose–response studies in our laboratory (8,22,23). Although the bolus injection of levosimendan is discouraged because of potential adverse drug effects, we adopted the aforementioned protocol of drug administration in this comparative study.
Hemodynamic and Oxygen Measurements
Heart rate, MAP, PAP, and central venous pressure were recorded continuously and analyzed at set intervals through hypoxia and reoxygenation and at every 30 min during the final 2 h of experimentation. Simultaneous blood gas analysis and plasma lactate levels were performed using an ABL 700 blood gas analyzer and an OSM3 Hemoximeter (Radiometer, Copenhagen, Denmark).
Biochemical Analysis and Histopathology
Porcine cardiac–specific troponin-I was evaluated from plasma frozen at baseline, 2 h of reoxygenation, and 2 h of drug treatment using enzyme-linked immunosorbent assay (no. 2010-4; Life Diagnostics, West Chester, PA).
Piglets were ultimately killed, and necropsy was immediately performed for collection of left ventricular and distal small intestinal tissue. Tissue was stored in 10% formalin for subsequent histological evaluation using scoring systems for H-R injury (31,32).
Additional myocardial and intestinal tissue was snap-frozen in liquid nitrogen and kept at −80 °C. Tissue lactate was determined using nicotinamide adenine dinucleotide enzyme-coupled colorimetric microplate assay as previously described (8,22). GSH and GSSG were measured using a commercially available glutathione assay kit (Cayman Chemical, Ann Arbor, MI, cat. no. 703002). Manufacturer’s instructions were followed with modifications and precautions to avoid auto-oxidation.
Statistics
SigmaPlot 11 software (Systat Software, San Jose, CA) was used for statistical analysis. Based on our previous experience, eight animals per group were needed for testing the primary hypothesis regarding hemodynamic effects. Data were analyzed with one-way and two-way repeated-measures analysis of variance or a Kruskal–Wallis test with post hoc pairwise analysis by Student-Newman-Keuls or Dunn’s method, respectively. To focus on drug effect after infusion, percentage change respective to predrug baseline was used to analyze data during the drug infusion phase (final 2 h). Correlations between variables were performed with Pearson moment or Spearman test as appropriate. Significance was defined as P < 0.05, and results are expressed as mean ± standard error of mean.
Statement of Financial Support
This project was funded by an operating grant (MOP53116) from the Canadian Institutes of Health Research. N.M. received support from the Clinician Investigator Program of the Royal College of Physicians and Surgeons of Canada.
Disclosure
Levosimendan was a gift from Abbott Inc. (Spain). The authors declare that they have no competing interests.
References
Shah P, Riphagen S, Beyene J, Perlman M . Multiorgan dysfunction in infants with post-asphyxial hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed 2004;89:F152–5.
Hankins GD, Koen S, Gei AF, Lopez SM, Van Hook JW, Anderson GD . Neonatal organ system injury in acute birth asphyxia sufficient to result in neonatal encephalopathy. Obstet Gynecol 2002;99(5 Pt 1):688–91.
Martín-Ancel A, García-Alix A, Gayá F, Cabañas F, Burgueros M, Quero J . Multiple organ involvement in perinatal asphyxia. J Pediatr 1995;127:786–93.
Subhedar NV . Treatment of hypotension in newborns. Semin Neonatol 2003;8:413–23.
Booker PD . Myocardial stunning in the neonate. Br J Anaesth 1998;80:371–83.
Seri I . Systemic and pulmonary effects of vasopressors and inotropes in the neonate. Biol Neonate 2006;89:340–2.
Wessel DL . Managing low cardiac output syndrome after congenital heart surgery. Crit Care Med 2001;29:10 Suppl:S220–30.
Joynt C, Bigam DL, Charrois G, Jewell LD, Korbutt G, Cheung PY . Intestinal hemodynamic effects of milrinone in asphyxiated newborn pigs after reoxygenation with 100% oxygen: a dose-response study. Shock 2009;31:292–9.
Joynt C, Bigam DL, Charrois G, Jewell LD, Korbutt G, Cheung PY . Milrinone, dobutamine or epinephrine use in asphyxiated newborn pigs resuscitated with 100% oxygen. Intensive Care Med 2010;36:1058–66.
Edes I, Kiss E, Kitada Y, et al. Effects of Levosimendan, a cardiotonic agent targeted to troponin C, on cardiac function and on phosphorylation and Ca2+ sensitivity of cardiac myofibrils and sarcoplasmic reticulum in guinea pig heart. Circ Res 1995;77:107–13.
Kopustinskiene DM, Pollesello P, Saris NE . Levosimendan is a mitochondrial K(ATP) channel opener. Eur J Pharmacol 2001;428:311–4.
DiSessa TG, Leitner M, Ti CC, Gluck L, Coen R, Friedman WF . The cardiovascular effects of dopamine in the severely asphyxiated neonate. J Pediatr 1981;99:772–6.
Chang AC, Atz AM, Wernovsky G, Burke RP, Wessel DL . Milrinone: systemic and pulmonary hemodynamic effects in neonates after cardiac surgery. Crit Care Med 1995;23:1907–14.
Ross-Ascuitto NT, Ascuitto RJ, Ramage D, McDonough KH . The effects of milrinone in the neonatal pig heart. Cardiovasc Drugs Ther 1991;5:1011–9.
Stocker CF, Shekerdemian LS, Nørgaard MA, et al. Mechanisms of a reduced cardiac output and the effects of milrinone and levosimendan in a model of infant cardiopulmonary bypass. Crit Care Med 2007;35:252–9.
McNamara PJ, Laique F, Muang-In S, Whyte HE . Milrinone improves oxygenation in neonates with severe persistent pulmonary hypertension of the newborn. J Crit Care 2006;21:217–22.
Segreti JA, Marsh KC, Polakowski JS, Fryer RM . Evoked changes in cardiovascular function in rats by infusion of levosimendan, OR-1896 [(R)-N-(4-(4-methyl-6-oxo-1,4,5,6-tetrahydropyridazin-3-yl)phenyl)acetamide], OR-1855 [(R)-6-(4-aminophenyl)-5-methyl-4,5-dihydropyridazin-3(2H)-one], dobutamine, and milrinone: comparative effects on peripheral resistance, cardiac output, dP/dt, pulse rate, and blood pressure. J Pharmacol Exp Ther 2008;325:331–40.
Egan JR, Clarke AJ, Williams S, et al. Levosimendan for low cardiac output: a pediatric experience. J Intensive Care Med 2006;21:183–7.
Namachivayam P, Crossland DS, Butt WW, Shekerdemian LS . Early experience with Levosimendan in children with ventricular dysfunction. Pediatr Crit Care Med 2006;7:445–8.
Braun JP, Schneider M, Kastrup M, Liu J . Treatment of acute heart failure in an infant after cardiac surgery using levosimendan. Eur J Cardiothorac Surg 2004;26:228–30.
Lechner E, Moosbauer W, Pinter M, Mair R, Tulzer G . Use of levosimendan, a new inodilator, for postoperative myocardial stunning in a premature neonate. Pediatr Crit Care Med 2007;8:61–3.
Joynt C, Bigam DL, Charrois G, Jewell LD, Korbutt G, Cheung PY . Dose-response effects of milrinone on hemodynamics of newborn pigs with hypoxia-reoxygenation. Intensive Care Med 2008;34:1321–9.
Esch J, Joynt C, Manouchehri N, et al. Differential Hemodynamic Effects of Levosimendan in a Porcine Model of Neonatal Hypoxia-Reoxygenation. Neonatology 2011;101:192–200.
Artman M, Kithas PA, Wike JS, Strada SJ . Inotropic responses change during postnatal maturation in rabbit. Am J Physiol 1988;255(2 Pt 2): H335–42.
Bassler D, Choong K, McNamara P, Kirpalani H . Neonatal persistent pulmonary hypertension treated with milrinone: four case reports. Biol Neonate 2006;89:1–5.
Danhaive O, Margossian R, Geva T, Kourembanas S . Pulmonary hypertension and right ventricular dysfunction in growth-restricted, extremely low birth weight neonates. J Perinatol 2005;25:495–9.
Gratton R, Carmichael L, Homan J, Richardson B. Carotid arterial blood flow in the bovine fetus as a continuous measure of cerebral blood flow. J Soc Gynecol Invest 1996; 3:60–65.
Borke WB, Munkeby BH, Halvorsen B, et al. Increased myocardial matrix metalloproteinases in hypoxic newborn pigs during resuscitation: effects of oxygen and carbon dioxide. Eur J Clin Invest 2004;34:459–66.
Markus T, Hansson S, Amer-Wåhlin I, Hellström-Westas L, Saugstad OD, Ley D . Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatr Res 2007;62:71–7.
Swindle M. Porcine models in surgical research 1985 An overview. In: Tumbleson M, ed. Swine in Biomedical Research, vol. 1. New York, NY: Plenum, 1985:235–242.
Rose AG, Opie LH, Bricknell OL . Early experimental myocardial infarction. Evaluation of histologic criteria and comparison with biochemical and electrocardiographic measurements. Arch Pathol Lab Med 1976;100:516–21.
Park PO, Haglund U, Bulkley GB, Fält K . The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery 1990;107:574–80.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manouchehri, N., Bigam, D., Churchill, T. et al. Milrinone is preferred to levosimendan for mesenteric perfusion in hypoxia-reoxygenated newborn piglets treated with dopamine. Pediatr Res 71, 241–246 (2012). https://doi.org/10.1038/pr.2011.48
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pr.2011.48
This article is cited by
-
Phase 1 study of two inodilators in neonates undergoing cardiovascular surgery
Pediatric Research (2013)