Abstract
Sustained lung inflations (SIs) immediately after birth might decrease the need for subsequent mechanical ventilation in preterm infants. However, effects of SIs on oxygenation and hemodynamics are undetermined. Our aim was to study immediate effects of SIs on heart rate, arterial oxygen saturation, and cerebral tissue oxygen saturation in preterm infants supported with SIs after birth for lung recruitment. Heart rate, arterial oxygen saturation, and cerebral tissue oxygen saturation using near infrared spectroscopy was measured in 24 preterm infants of 28.0 (26.6–29.3) wk GA [median (interquartile range)] during resuscitation using up to three SIs of 20, 25, and 30 cm H2O of 15 s duration each followed by nasal continuous positive airway pressure (CPAP) as first line approach for respiratory support. During positioning and suctioning immediately after delivery infants became progressively hypoxemic and bradycardic before respiratory support was initiated. In 18 infants (75%), more than one SI were applied. During the last SIs, there was a rapid increase in the infants' heart rate and an increase in cerebral tissue oxygen saturation. Arterial saturation increased with slight delay. In conclusion, effective last sustained inflations increase heart rate and cerebral tissue oxygen saturation to be followed by an increase in arterial saturation.
Similar content being viewed by others
Main
Replacement of amniotic fluid by air to establish a gaseous functional residual capacity (FRC) is essential for successful transition after birth (1,2). Term and preterm infants begin to breathe after delivery with deep inspirations and braking of expirations (3–7). Closure of the larynx during expiration may help the newborn to maintain FRC (6,8).
Clinical and laboratory observations have shown that the application of continuous positive airway pressure (CPAP) helps to establish a gaseous FRC and improve gas exchange (9–11). In a preterm animal model, the application of SIs of 10 to 20 s duration did further enhance movement of amniotic fluid into the distal airways resulting in an increased FRC and more uniform lung aeration than CPAP alone (10,12).
Clinical studies suggest that the use of SIs in preterm infants may help to reduce the need for intubation and mechanical ventilation without adverse effects (13,14). A lower rate of bronchopulmonary dysplasia (BPD) was reported in one study when SIs followed by nasal CPAP was compared with bag and mask ventilation (13). However, these trials have limited power to prove safety of SIs.
Possible sequelae of SIs include overdistension of the lung and compromise of hemodynamics as a result of the impaired systemic venous return and decreased pulmonary blood flow caused by the increase in intrathoracic pressure during the procedure (15–18). Cerebral blood flow may be impaired resulting in brain damage in the preterm infant (19–21). Impaired cerebral venous flow may predispose to intraventricular hemorrhage (IVH) (22) or periventricular hemorrhagic infarction (23,24).
Near-infrared spectroscopy (NIRS) has been used to measure regional cerebral tissue oxygen saturation (rcSO2) in term and preterm infants (19,25–30). rcSO2 seems related to both arterial saturation and cerebral blood flow (25,31,32). The aim was to study immediate hemodynamic effects of SIs as measured by heart rate, arterial oxygen saturation (SpO2), and rcSO2 applied after delivery in very low birth weight (VLBW) infants.
METHODS
This prospective observational study was approved by the local ethics committee (Ethikkommission, University of Ulm, Germany; No. 49/08). Written informed consent was obtained from all parents before or after birth in agreement with the decision of the ethics committee, which included a waiver for this specific situation. Infants <1500 g born between December 2009 and August 2010 at the University Hospital of Ulm were eligible for this study if respiratory support was indicated and a research team member who was not involved in the care for the infant was present.
Resuscitation was performed according to a local standardized protocol that includes SIs as first line respiratory support as described before (14,33). Neonatal resuscitation was performed under an overhead warmer. A F120 respirator (Stephan, Gackenbach, Germany) was used for respiratory support. All infants were wrapped into a plastic bag to avoid evaporative heat loss and positioned supine. A sensor for rcSO2 monitoring (Foresight, Casmed, Branford, CT) was placed at the center of the infant's forehead and fixed with adhesive tape. Sensors for pulse oximetry (Radical, Masimo, Irvine, CA) were applied on the right hand and left leg. Pulse oximeter settings were 2 s averaging time and maximum sensitivity. After gentle suctioning of excess amniotic fluid from the mouth, a nasopharyngeal tube was inserted at 3–4 cm, and CPAP of 5 cm H2O was applied with a fraction of inspired oxygen (FiO2) of 0.4. If the infant had no respiratory activity, the heart rate remained below 100 beats per minute (bpm) and/or SpO2 did not increase within ∼15 s a SI of 15 s duration was applied at a pressure of 20 cm H2O by pressing the inflation hold of the F120 respirator while closing the infant's mouth and nostrils gently. If the heart rate remained below 100/bpm and/or SpO2 remained <70% without further increase the SI was repeated after ∼15 s up to two times using pressures of 25 and 30 cm H2O, respectively. In this study, a SI that was repeated at a higher pressure is called “ineffective” SI. The last SI applied was called “effective last” SI. This SI was frequently accompanied by an increase in heart rate >100 bpm and followed by an increase in SpO2. Thereafter, nasal CPAP or nasal intermittent mandatory ventilation was continued at the discretion of the clinical team at a FiO2 adjusted to maintain a preductal SpO2 at 80–92%. Criteria for intubation and invasive ventilation after SIs were FiO2 ≥0.4, persistent heart rate <100/bpm, or a GA <25 wk for prophylactic surfactant therapy. Chest compressions would have been applied if bradycardia <60 bpm would have persisted after intubation and MV. Serial data of the Radical pulse oximeter and Foresight cerebral oximeter were recorded in 2 s intervals simultaneously, and data were processed in Excel (Microsoft, Redmond, WA).
Statistical analysis.
Medians and interquartile ranges are given. Data were analyzed using ANOVA, ANOVA on ranks for repeated measurements or paired t test, where appropriate. Mean values of variables of interest measured during the 15-s interval before a SI were compared with data obtained during SI and with data obtained during the 15-s interval after the SI. Values at the beginning of the SIs were compared with values at the end of the SIs. Data were analyzed with SigmaStat V2.03 (Systat Software, San Jose, CA).
RESULTS
During the study period, 88 infants <1500 g birth weight were born at our unit with 27.1 (25.2–29.0) wk GA and 860 (708–1106) g birth weight. Of these infants, 80 (91%) were delivered by cesarean section and 75 (85%) received SIs for respiratory support. rcSO2 was measured in 36 infants (41%). Twelve infants were excluded, because rcSO2 signal was not available before SIs were performed (n = 9), or because no SI was necessary for respiratory support (n = 3).
Twenty-four VLBW infants were included in the analysis. Characteristics of infants are summarized in Table 1. All study infants received a SI of 20 cm H2O of 15 s duration 40 (30–51) s after the beginning of monitoring. Sixteen infants received a second SI of 25 cm H2O 21 (16–26) s after the end of the first inflation, and two infants received a third inflation of 30 cm H2O after another 24 and 32 s. Twenty-two infants were stabilized with early nasal CPAP in the delivery room finally. Two infants were intubated because of persistent high FiO2 for >10 min after respiratory support was initiated. No infant received chest compressions.
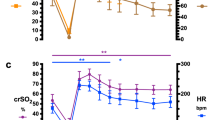
Time from birth to the beginning of monitoring was 52 (40–54) s. At the beginning of monitoring, the heart rate was 109 (85–130) bpm, the preductal SpO2 was 51% (41–56%), and the postductal SpO2 was 36% (30–49%). While wrapping the infants into a plastic bag, suctioning, and application of a nasopharyngeal tube for CPAP, the heart rate decreased to a minimum of 65 (58–84) bpm, the minimal preductal SpO2 was 41% (30–54%), and the postductal SpO2 was 21% (10–40%). With respiratory support including sustained inflations, the heart rate increased to >100 bpm within 56 s after beginning of the monitoring period. By 1 min, the preductal SpO2 was 50% (39–60%), reached 81% (63–90%) by 2 min and continued to rise to a median of 90% (86–92%) at 10 min (Fig. 1A, B). At the beginning of monitoring, rcSO2 was 36% (31–47%) and increased to 40% (33–51%)% at 1 min, 64% (54–70%) at 2 min, and reached a steady state of ∼76–80% after around 4 min (Fig. 1C).
To analyze the immediate hemodynamic effects of the SIs in more detail, heart rate, preductal SpO2, and rcSO2 were analyzed in SIs that were repeated at a higher pressure for clinical reasons (called “ineffective SI”) and in SIs that were not repeated (called “effective last” SI) separately. Data obtained during SIs were compared with data obtained immediately before and after SIs. Ineffective SIs did not affect heart rate (p = 0.31), SpO2 (p = 0.18), or rcSO2 (p = 0.38; Fig. 2A–C). During effective last SIs, there was a rapid increase in heart rate from 77 (65–117) bpm at the beginning of the effective last SIs to 124 (92–150) bpm at the end of the effective last SIs (p < 0.001) associated with a slow increase in SpO2 (p < 0.01) and rcSO2 (p < 0.001). After the effective last SIs, SpO2 increased rapidly (Fig. 2D – F). Short-term outcomes of the infants are given in Table 2.
Effects of sustained inflations on heart rate, SpO2, and rcSO2. Heart rate (A and D), preductal SpO2 (B and E), and rcSO2 (C and F) are shown separately for ineffective SIs, which were repeated at a higher pressure (A–C) or effective last SIs (D–F). The horizontal bars labeled SI indicate the period of SIs. Medians and interquartile ranges are given. (§ = NS; * = p < 0.01; ** = p < 0.001.).
DISCUSSION
To our knowledge, this is the first report on rcSO2 as measured by NIRS in VLBW infants during the first minutes after birth. Recently, data on rcSO2 have become available in healthy term infants during immediate postnatal adaptation (34–36). In these infants, cerebral oxygenation rose continuously and reached a plateau after around 7–8 min after birth without any support of respiration (34,36). In this study, rcSO2 remained low and heart rate and SpO2 continued to decrease until respiratory support was initiated. The increase in heart rate and SpO2 with respiratory support including application of SIs for lung recruitment seems similar to the increase observed in infants <30 wk GA where intermittent positive pressure ventilation or bag and mask ventilation had to be applied in most infants (37).
The long-time constant of the newborn lung filled with amniotic fluid suggests that the use of a SI may be a better approach to create a gaseous FRC than the use of short breaths (12). In preterm rabbits, SIs of 20-s duration were shown to achieve a larger gas volume without total or regional lung over inflation compared with 10-s SIs or short breaths (10,12). By phase-contrast x-ray, it was demonstrated that about 15 s of SI are required for uniform lung aeration in these animals (12). This corresponds well with the duration of SIs used in our delivery room clinically.
However, SIs have been associated with adverse hemodynamic effects in various adult animal models (15–17). Increased intrathoracic pressure may impair venous return to the right heart, and alveolar overdistension may compress of perialveolar capillaries resulting in an increase in pulmonary vascular resistance and compromised left ventricular venous return and cardiac output. Decreased pulmonary blood flow, transient hypotension, and low cardiac output during periods of increased intrathoracic pressure have been demonstrated (15–17). The magnitude of these effects was dependent on the inflation pressure applied and on the mechanical characteristics of the lung with less interference in the more severely injured lung with low lung compliance (15).
Severe hemodynamic effects of SIs are less likely to occur in the newborn. Before birth, pulmonary vascular resistance is high, and systemic cardiac output is maintained to a large part by right to left shunting at the ductus arteriosus and at the atrial level. In the first minutes after birth, right to left shunting persists as evidenced by the difference in pre- and postductal SpO2 found in our infants in the first minutes after birth. This might protect the systemic arterial circulation if pulmonary vascular resistance is increased during SIs. The fact that in the preterm sheep animal model, the mean arterial pressure, the heart rate, and the central venous pressure were not altered by a SI at 15 min after birth in contrast to a SI applied 4 h later supports this consideration (38).
Direct measurements of cardiac output and cerebral blood flow are not possible during immediate neonatal resuscitation of preterm infants. However, NIRS allows estimation of cerebral hemodynamics by investigation of rcSO2 noninvasively. rcSO2 represents the mixed oxygen saturation in a multicompartmental system of arteries, arterioles, capillaries, venules, and veins. The cerebral venous blood volume fraction contributes ∼70–75% to rcSO2 at normoxia (39). rcSO2 is affected by several factors: arterial saturation, cerebral blood flow, cerebral blood volume, and cerebral oxygen consumption (40). Recent animal studies indicate correlation of the rcSO2 with cerebral blood flow under stable experimental conditions with high sensitivity to variations in cerebral blood flow at 0.1 Hz and below (32,41). In clinical studies in preterm infants, there was positive correlation of rcSO2 with superior vena cava flow of which ∼80% is estimated to be venous return from the brain (25,31). Therefore, we expect that a clinically relevant impairment of cerebral blood flow together with an impaired cerebral venous return during a SI should be detectable by NIRS measurement, even in the situation of unstable SpO2 and unknown brain metabolism during neonatal resuscitation. Nevertheless, accuracy of the rcSO2 and SpO2 in the situation of severe hypoxemia may be impaired. We did not observe a decrease of rcSO2 during ineffective or effective SIs in our VLBW infants. Therefore, as the arterial SpO2 remained relative stable during SIs, a relevant decrease of cerebral blood flow seems unlikely during SIs.
Nine infants had to be excluded from analysis, because the rcSO2 signal was available only after SIs were applied. Although not reaching statistical significance, these infants were smaller [710 (545–905) g] and less mature [25.9 (24.3–28.3) wk GA]. We cannot rule out bias from excluding these infants. However, clinically, even the smallest premature infants seem to respond to SIs similar to more mature VLBW infants.
Because of the differences in the lung mechanics, different pressures might be necessary to aerate the lung in individual infants. Therefore, our local protocol suggests to apply the first SI at a lower pressure to avoid overdistension of the lung and to increase the pressure for each subsequent SI to aerate the lung (14,33) resulting in increasing SpO2 and heart rate.
The first SI at 20 cm H2O was considered ineffective in 75% of infants by the clinical team because heart rate and SpO2 remained unchanged during and after the SIs. Ineffective SIs might prolong the time until effective ventilation, and in four infants the heart rate continued to fall during ineffective SIs. However, rcSO2 remained stable in these infants.
Finally, the heart rate and saturation increased in timely association with the second SI at a pressure of 25 cm H2O in 16 infants and with a third SI of 30 cm H2O in two infants. The inflation pressures we finally used are little higher than opening pressures observed in preterm infants at a median age of 3 h during high frequency oscillation ventilation (HFOV) before surfactant therapy (42). In this study, temporary recruitment at these pressures was tolerated without clinically relevant hemodynamic compromise (42,43).
The tremendous increase in heart rate observed during effective last SIs before cessation of hypoxemia is surprising. Several mechanisms might be responsible for the bradycardia after birth. First, the direct effect of hypoxemia on cardiac musculature (44). Second, bradycardia might be the result of a vagal reflex secondary to low left and right atrial venous return after cord clamping (45) and to iatrogenic maneuvers like suctioning and nasopharyngeal tube insertion. During resuscitation clinically, the normalization of the heart rate seems to indicate aeration of the lung and recovery of the infant. Eventually, oxygenated blood may enter the coronary arteries before the increase of SpO2 can be measured at the peripheral level and by this normalize the heart rate. In addition, reflectory vagal inhibition induced by moderate lung inflation resulting in acceleration of the heart rate has been demonstrated in animal experiments (46,47). Finally, we cannot rule out spontaneous recovery, eventually enhanced by previous ineffective SIs. The increase in heart rate was accompanied by increasing rcSO2. We speculate, that this is the result of a higher cardiac output and cerebral blood flow at a higher heart rate.
So far, two randomized trials compared a delivery room approach including the use of SIs with standard treatment (13,14). In addition, two retrospective studies compared the use of SIs in the delivery room with standard treatments of historic cohorts (33,48). These studies included 302 infants treated with SIs. The use of SIs seems to be associated with a decreased need for mechanical ventilation and BPD. No increased rate of IVH was associated with the application of SIs after birth in these infants (13,14,33,48). However, because of the retrospective design of two studies and the limited power of the two randomized trials despite the reasonable sample size, a small but clinically relevant adverse effect on the rate of IVH and neurodevelopmental outcome cannot be excluded. Our results imply that the application of SIs in VLBW infants is not associated with decreasing rcSO2, but with increasing heart rate and increasing rcSO2 if performed at an effective pressure. These data might be helpful for initiation of a randomized trial to compare the effects of SIs with other modes of respiratory support powered for relevant outcome measures such as BPD or even better survival without neurodevelopmental impairment.
In conclusion, we were able to show that during effective last SIs, the heart rate and rcSO2 increased, followed by an rapid increase in SpO2. A large clinical trial is needed to evaluate the effects of SIs in comparison with other modes of delivery room respiratory support on long-term outcome measures.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- bpm:
-
beats per minute
- CPAP:
-
continuous positive airway pressure
- FiO2:
-
fraction of inspired oxygen
- FRC:
-
functional residual capacity
- IVH:
-
intraventricular hemorrhage
- NIRS:
-
near-infrared spectroscopy
- rcSO2:
-
regional cerebral tissue oxygen saturation
- SIs:
-
sustained lung inflations
- SpO2:
-
arterial oxygen saturation
- VLBW:
-
very low birth weight
References
Hooper SB, Kitchen MJ, Siew ML, Lewis RA, Fouras A, te Pas AB, Siu KK, Yagi N, Uesugi K, Wallace MJ 2009 Imaging lung aeration and lung liquid clearance at birth using phase contrast X-ray imaging. Clin Exp Pharmacol Physiol 36: 117–125
te Pas AB, Davis PG, Hooper SB, Morley CJ 2008 From liquid to air: breathing after birth. J Pediatr 152: 607–611
Vyas H, Field D, Milner AD, Hopkin IE 1986 Determinants of the first inspiratory volume and functional residual capacity at birth. Pediatr Pulmonol 2: 189–193
Vyas H, Milner AD, Hopkins IE 1981 Intrathoracic pressure and volume changes during the spontaneous onset of respiration in babies born by cesarean section and by vaginal delivery. J Pediatr 99: 787–791
Kosch PC, Stark AR 1984 Dynamic maintenance of end-expiratory lung volume in full-term infants. J Appl Physiol 57: 1126–1133
te Pas AB, Wong C, Kamlin CO, Dawson JA, Morley CJ, Davis PG 2009 Breathing patterns in preterm and term infants immediately after birth. Pediatr Res 65: 352–356
te Pas AB, Davis PG, Kamlin CO, Dawson J, O'Donnell CP, Morley CJ 2008 Spontaneous breathing patterns of very preterm infants treated with continuous positive airway pressure at birth. Pediatr Res 64: 281–285
Milner AD, Saunders RA, Hopkin IE 1978 Is air trapping important in the maintenance of the functional residual capacity in the hours after birth?. Early Hum Dev 2: 97–105
Lindner W, Pohlandt F 2007 Oxygenation and ventilation in spontaneously breathing very preterm infants with nasopharyngeal CPAP in the delivery room. Acta Paediatr 96: 17–22
te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, Yagi N, Uesugi K, Donath S, Davis PG, Morley CJ, Hooper SB 2009 Establishing functional residual capacity at birth: the effect of sustained inflation and positive end-expiratory pressure in a preterm rabbit model. Pediatr Res 65: 537–541
Siew ML, te Pas AB, Wallace MJ, Kitchen MJ, Lewis RA, Fouras A, Morley CJ, Davis PG, Yagi N, Uesugi K, Hooper SB 2009 Positive end-expiratory pressure enhances development of a functional residual capacity in preterm rabbits ventilated from birth. J Appl Physiol 106: 1487–1493
te Pas AB, Siew M, Wallace MJ, Kitchen MJ, Fouras A, Lewis RA, Yagi N, Uesugi K, Donath S, Davis PG, Morley CJ, Hooper SB 2009 Effect of sustained inflation length on establishing functional residual capacity at birth in ventilated premature rabbits. Pediatr Res 66: 295–300
te Pas AB, Walther FJ 2007 A randomized, controlled trial of delivery-room respiratory management in very preterm infants. Pediatrics 120: 322–329
Lindner W, Hogel J, Pohlandt F 2005 Sustained pressure-controlled inflation or intermittent mandatory ventilation in preterm infants in the delivery room? A randomized, controlled trial on initial respiratory support via nasopharyngeal tube. Acta Paediatr 94: 303–309
Lim SC, Adams AB, Simonson DA, Dries DJ, Broccard AF, Hotchkiss JR, Marini JJ 2004 Transient hemodynamic effects of recruitment maneuvers in three experimental models of acute lung injury. Crit Care Med 32: 2378–2384
Muellenbach RM, Kredel M, Zollhoefer B, Wunder C, Roewer N, Brederlau J 2006 Sustained inflation and incremental mean airway pressure trial during conventional and high-frequency oscillatory ventilation in a large porcine model of acute respiratory distress syndrome. BMC Anesthesiol 6: 8
Odenstedt H, Aneman A, Karason S, Stenqvist O, Lundin S 2005 Acute hemodynamic changes during lung recruitment in lavage and endotoxin-induced ALI. Intensive Care Med 31: 112–120
Björklund LJ, Ingimarsson J, Curstedt T, John J, Robertson B, Werner O, Vilstrup CT 1997 Manual ventilation with a few large breaths at birth compromises the therapeutic effect of subsequent surfactant replacement in immature lambs. Pediatr Res 42: 348–355
Sorensen LC, Maroun LL, Borch K, Lou HC, Greisen G 2008 Neonatal cerebral oxygenation is not linked to foetal vasculitis and predicts intraventricular haemorrhage in preterm infants. Acta Paediatr 97: 1529–1534
Osborn DA, Evans N, Kluckow M, Bowen JR, Rieger I 2007 Low superior vena cava flow and effect of inotropes on neurodevelopment to 3 years in preterm infants. Pediatrics 120: 372–380
Meek JH, Tyszczuk L, Elwell CE, Wyatt JS 1999 Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed 81: F15–F18
Ghazi-Birry HS, Brown WR, Moody DM, Challa VR, Block SM, Reboussin DM 1997 Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR Am J Neuroradiol 18: 219–229
Dudink J, Lequin M, Weisglas-Kuperus N, Conneman N, van Goudoever JB, Govaert P 2008 Venous subtypes of preterm periventricular haemorrhagic infarction. Arch Dis Child Fetal Neonatal Ed 93: F201–F206
Takashima S, Mito T, Ando Y 1986 Pathogenesis of periventricular white matter hemorrhages in preterm infants. Brain Dev 8: 25–30
Moran M, Miletin J, Pichova K, Dempsey EM 2009 Cerebral tissue oxygenation index and superior vena cava blood flow in the very low birth weight infant. Acta Paediatr 98: 43–46
Lemmers PM, Toet M, van Schelven LJ, van Bel F 2006 Cerebral oxygenation and cerebral oxygen extraction in the preterm infant: the impact of respiratory distress syndrome. Exp Brain Res 173: 458–467
Baerts W, Lemmers PM, van Bel F 2011 Cerebral oxygenation and oxygen extraction in the preterm infant during desaturation: effects of increasing FiO(2) to assist recovery. Neonatology 99: 65–72
Lemmers PM, Toet MC, van Bel F 2008 Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 121: 142–147
Verhagen EA, ter Horst HJ, Keating P, Martijn A, Van Braeckel KN, Bos AF 2010 Cerebral oxygenation in preterm infants with germinal matrix-intraventricular hemorrhages. Stroke 41: 2901–2907
van Hoften JC, Verhagen EA, Keating P, ter Horst HJ, Bos AF 2010 Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed 95: F352–F358
Takami T, Sunohara D, Kondo A, Mizukaki N, Suganami Y, Takei Y, Miyajima T, Hoshika A 2010 Changes in cerebral perfusion in extremely LBW infants during the first 72 h after birth. Pediatr Res 68: 435–439
Wong FY, Nakamura M, Alexiou T, Brodecky V, Walker AM 2009 Tissue oxygenation index measured using spatially resolved spectroscopy correlates with changes in cerebral blood flow in newborn lambs. Intensive Care Med 35: 1464–1470
Lindner W, Vossbeck S, Hummler H, Pohlandt F 1999 Delivery room management of extremely low birth weight infants: spontaneous breathing or intubation?. Pediatrics 103: 961–967
Fauchère JC, Schulz G, Haensse D, Keller E, Ersch J, Bucher HU, Wolf M 2010 Near-infrared spectroscopy measurements of cerebral oxygenation in newborns during immediate postnatal adaptation. J Pediatr 156: 372–376
Isobe K, Kusaka T, Fujikawa Y, Kondo M, Kawada K, Yasuda S, Itoh S, Hirao K, Onishi S 2000 Changes in cerebral hemoglobin concentration and oxygen saturation immediately after birth in the human neonate using full-spectrum near infrared spectroscopy. J Biomed Opt 5: 283–286
Urlesberger B, Grossauer K, Pocivalnik M, Avian A, Muller W, Pichler G 2010 Regional oxygen saturation of the brain and peripheral tissue during birth transition of term infants. J Pediatr 157: 740–744
Dawson JA, Kamlin CO, Wong C, te Pas AB, O'Donnell CP, Donath SM, Davis PG, Morley CJ 2009 Oxygen saturation and heart rate during delivery room resuscitation of infants <30 weeks' gestation with air or 100% oxygen. Arch Dis Child Fetal Neonatal Ed 94: F87–F91
Klöpping-Ketelaars WA, Maertzdorf WJ, Blanco CE 1994 Cardiovascular changes during sustained lung inflations in premature newborn lambs. Acta Paediatr 83: 897–902
Wong FY, Alexiou T, Samarasinghe T, Brodecky V, Walker AM 2010 Cerebral arterial and venous contributions to tissue oxygenation index measured using spatially resolved spectroscopy in newborn lambs. Anesthesiology 113: 1385–1391
van Bel F, Lemmers P, Naulaers G 2008 Monitoring neonatal regional cerebral oxygen saturation in clinical practice: value and pitfalls. Neonatology 94: 237–244
Elliott JT, Diop M, Tichauer KM, Lee TY, St Lawrence K 2010 Quantitative measurement of cerebral blood flow in a juvenile porcine model by depth-resolved near-infrared spectroscopy. J Biomed Opt 15: 037014
De Jaegere A, van Veenendaal MB, Michiels A, van Kaam AH 2006 Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am J Respir Crit Care Med 174: 639–645
de Waal K, Evans N, van der Lee J, van Kaam A 2009 Effect of lung recruitment on pulmonary, systemic, and ductal blood flow in preterm infants. J Pediatr 154: 651–655
Reynolds SR, Paul WM 1958 Relation of bradycardia and blood pressure of the fetal lamb in utero to mild and severe hypoxia. Am J Physiol 193: 249–256
Dawson JA, Kamlin CO, Wong C, te Pas AB, Vento M, Cole TJ, Donath SM, Hooper SB, Davis PG, Morley CJ 2010 Changes in heart rate in the first minutes after birth. Arch Dis Child Fetal Neonatal Ed 95: F177–F181
De Daly MB, Scott MJ 1958 The effects of stimulation of the carotid body chemoreceptors on heart rate in the dog. J Physiol 144: 148–166
Anrep GV, Pascual W, Roessler R 1936 Respiratory variations of the heart rate. II. The central mechanism of the respiratory arrhythmia and the inter-relations between the central and the reflex mechanisms. Proc R Soc Lond B Biol Sci 119: 218–230
Lista G, Fontana P, Castoldi F, Cavigioli F, Dani C 2011 Does SI at birth improve outcome of preterm infants at risk for respiratory distress syndrome?. Neonatology 99: 45–50
Acknowledgements
We thank Bob Kopotic for very useful discussions and his expert technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuchs, H., Lindner, W., Buschko, A. et al. Cerebral Oxygenation in Very Low Birth Weight Infants Supported With Sustained Lung Inflations After Birth. Pediatr Res 70, 176–180 (2011). https://doi.org/10.1203/PDR.0b013e318220c1e0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/PDR.0b013e318220c1e0
This article is cited by
-
Fetal to neonatal transition: what additional information can be provided by cerebral near infrared spectroscopy?
Pediatric Research (2022)
-
The SURV1VE trial—sustained inflation and chest compression versus 3:1 chest compression-to-ventilation ratio during cardiopulmonary resuscitation of asphyxiated newborns: study protocol for a cluster randomized controlled trial
Trials (2019)
-
Cerebral regional tissue Oxygen Saturation to Guide Oxygen Delivery in preterm neonates during immediate transition after birth (COSGOD III): an investigator-initiated, randomized, multi-center, multi-national, clinical trial on additional cerebral tissue oxygen saturation monitoring combined with defined treatment guidelines versus standard monitoring and treatment as usual in premature infants during immediate transition: study protocol for a randomized controlled trial
Trials (2019)
-
Achieving and maintaining lung volume in the preterm infant: from the first breath to the NICU
European Journal of Pediatrics (2017)
-
Pressure- versus volume-limited sustained inflations at resuscitation of premature newborn lambs
BMC Pediatrics (2014)