Abstract
The objectives of this study were to evaluate the differences in whole brain white matter (WM) volume and anisotropy between preterm and term children and to determine the relationships with cognitive outcome. Twenty-five low birth weight (BW), preterm, neurologically normal children between 8.8 and 11.5 y of age were recruited for volumetric and diffusion-tensor magnetic resonance imaging (DTI), together with 13 age-matched term control subjects. Subsequent intelligence quotient (IQ) testing was performed for 21 preterm children within 6 mo of imaging studies. We computed the mean volume and fractional anisotropy (FA) of the whole brain WM and compared the differences between the two groups. Mean WM volume and FA were significantly lower in the preterm group (p = 0.014 and p < 0.001, respectively). Multiple regression analysis found both WM volume and FA to be independent variables significantly affecting full scale IQ (FSIQ) (r2 = 0.407, p = 0.021 and r2 = 0.496, p = 0.005, respectively) after adjusting for BW, gestational age (GA), and gender. In the evaluation of the whole brain WM of preterm children, we found that both volume and FA remain reduced at late childhood with both parameters significantly affecting long-term cognitive outcome.
Similar content being viewed by others
Main
Nearly 90% of very low BW (VLBW) premature infants now survive the neonatal period due to major advances in neonatal intensive care (1). However, approximately 10% of VLBW preterm infants later exhibit cerebral palsy, 30%–50% later manifest neurodevelopmental handicap during their preschool years, and 50% require special help in grade school. Even in the absence of global intellectual deficits, premature infants are at increased risk of learning disabilities, academic difficulties (2,3), and behavioral problems (4,5). Nearly 20% repeat a grade in school by age 8 y (6).
With recent advances in magnetic resonance imaging (MRI), subtle brain anomalies are now described in preterm children using qualitative and quantitative MR methods, such as decrease in cortical complexity (7) and decreases in total cortical gray matter (GM) (8–10) and WM volume (11–13), as well as regional reduced tissue volume (14–16). Furthermore, an association has been found between some of these abnormalities, such as delay in gyral development being highly related to the presence and severity of WM abnormalities (17). To date, WM volumetric studies in preterm infants and children have demonstrated total WM volume reduction to be significantly correlated with BW (11) and GA (12,18), and some studies further show the adverse effect of regional WM changes on neurocognitive function (19–21).
DTI is a relatively new quantitative MRI technique advantageous for the evaluation of the WM fibers in the brain because it is able to evaluate the directional variability of water diffusion. In WM tracts, the movement of water molecules is relatively free in the direction parallel to its length but restricted in the perpendicular directions by barriers such as axonal membrane and myelin sheaths surrounding it (22). This property, termed diffusion anisotropy, can be quantified by the index derived from DTI, FA. Although axonal membrane is implicated to be the primary source of anisotropy, the degree of anisotropy can be modulated by myelination (22). DTI can therefore be used to study the macro- and microstructure of WM and also estimate myelination and WM maturation in vivo. This technique is therefore potentially more sensitive for the detection of WM diseases than conventional MRI.
Developmental changes in the preterm brain have been demonstrated using DTI (23,24). With increasing GA, WM becomes increasingly anisotropic (25). DTI has also been used to elucidate differences in the development of WM in preterm infants compared with term infants. Premature newborns with evidence of early WM injury (WMI) on conventional imaging are found to have lower FA in the central WM and internal capsule when reassessed at term (26). Another study showed that premature newborns with WMI on conventional MRI do not have the expected increase in FA at multiple WM sites at term (27). These effects on WM FA have been found to persist into the teenage years (28,29). Thus, these studies indicate that injury related to premature birth has deleterious effects on the development of WM.
In a cohort of VLBW preterm children, we studied the quantitative imaging parameters of cerebral WM by volumetric studies and DTI, comparing with term normal age-matched controls. We hypothesized that mean WM volume and anisotropy are reduced in children who are born preterm with VLBW compared with controls. We also evaluated whether these parameters of WM damage correlate with cognitive outcome.
METHODS
The study was approved by the hospital institutional review board and informed written consent was obtained from the controls, patients, and parents.
Subjects.
We randomly recruited 25 subjects born prematurely from the VLBW registry that was set up in Hong Kong between January 1993 and December 1995. All infants with BW <1500 g who were discharged from public hospitals were enrolled in the registry. A total of 210 infants were enrolled.
Inclusion criteria for premature subjects was GA <37 wk, BW <1500 g, good past health apart from prematurity, and neurologically normal with the ability to cooperate during the MRI scan by keeping still for 20 min. The last inclusion criterion was included because we believed that it was ethically not justifiable to sedate children for this study. Therefore, this limited the study subjects to those with no major cognitive or behavioral deficits.
Children with congenital brain malformations or syndromes or congenital brain infection were excluded.
Thirteen age-matched term control subjects selected from a preexisting database were recruited for comparison. All control subjects were neurologically normal with GA >37 wk and BW >2500 g. The control subjects were also matched for socioeconomic status and educational level.
Neurodevelopmental and cognitive assessments.
The preterm cohort was invited to return for cognitive function assessment by a clinical psychologist using the Hong Kong Wechsler Intelligence Scale for Children (HKWISC) and a full neurologic examination by a pediatric neurologist within 6 mo after MRI study. The HKWISC is a norm-referenced instrument for assessing the intellectual function of children aged 5 y 0 mo through 15 y 11 mo. It provides a full-scale intelligence quotient (FSIQ) with a mean of 100 and a SD of 15. A FSIQ of 90–109 is considered as average intelligence, whereas an FSIQ of 70–79 is considered limited intelligence and an FSIQ of 80–89 as low average intelligence (30).
Perinatal risk factors, including BW and GA, were recorded from the case notes.
MRI studies.
MRI was performed using a Signa 1.5-Tesla imager (General Electric Medical Systems, Milwaukee, WI) with a standard head coil. The following sequences were performed in all patients: axial spin-echo T1-weighted, fast spin-echo proton density, and T2-weighted images, coronal fluid-attenuated inversion recovery sequences, three-dimensional spoiled gradient recalled (3DSPGR) images (slice thickness = 3 mm with no gap of the whole brain, TR/TE/TI = 11.3/4.2/600 ms, acquisition matrix = 256 × 256, flip angle = 15 degrees, field of view = 23 cm), DTI (echo planar imaging, TR/TE = 10,000/84 ms, b factor = 1200 s/mm (2), diffusion-weighted images in 25 noncollinear directions with one nondiffusion-weighted (b0) image, acquisition matrix = 128 × 128, field of view = 28 cm, slice thickness = 5 mm with 1.5-mm gap, imaging time approximately 5 min). The diffusion-weighted MR images were transferred to a workstation (Advantage Workstation, GE Medical Systems) for data processing using a commercial software program (FUNCTOOL; GE Medical Systems) and FA maps were generated accordingly.
Image analysis.
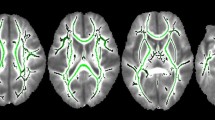
All image manipulations were performed using SPM2 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, UK) and MATLAB 6.5 (The MathWorks, Inc., Natick, MA). In SPM2, segmentation of image not normalized to standard space involves three steps: (1) the image is normalized to standard space using an appropriate template, (2) the normalized image is segmented in standard space, and (3) the output of the second step is transformed back to the space of the original image. In our analysis, we performed the image processing in two phases. In the first phase, we created customized 3DSPGR and b0 templates because the use of customized templates would improve the accuracy of the normalization process. We performed this by linearly normalizing our subject's 3DSPGR and b0 images to the pediatric T1-weighted template CCHMC2_fp (Cincinnati Children's Hospital Medical Center, Cincinnati, OH) (31). In the second phase, the original 3D SPGR and b0 images were segmented using the respective customized templates created in phase 1 to parcel images into GM, WM, and cerebrospinal fluid (CSF). WM volume was calculated by adding up the probability values of all voxels of a 3DSPGR WM segment and multiplying the total by the volume of one voxel. Inherently registered with FA image, segmentation outputs from the b0 image were used to create a WM mask by evaluating the following formula (I_WM>I_GM)&(I_WM>I_CSF)&[I_WM>(1-I_GM-I_WM-I_CSF)] using ImCalc function in SPM2, and the resulting mask was subsequently used to calculate the mean FA value of the WM. Figure 1A shows a 3DSPGR image superimposed with the contour of its WM segmentation output. Figure 1B shows the WM mask derived from segmentation output of the b0 image used to map out the WM region in the FA map. For more details on the image processing, please refer to our previous publication (32).
Statistical analysis.
We computed the mean WM volume and FA and compared the differences between the two groups using a t test. We further evaluated the gender effect on WM volume and FA using a t test. Spearman's correlation, followed by multiple regression analyses, was performed to determine the association between WM volume, FA, gender, BW, GA, and FSIQ in the preterm group.
RESULTS
Preterm patients.
Twenty-five preterm children (14 males, 11 females) aged 8.8 to 11.5 y (mean age, 10.14 y, SD = 0.76 y) were included. BW and GA were 1141.6 ± 213.9 g (mean ± SD) and 29.4 ± 3.1 wk (mean ± SD), respectively. Except for three subjects who had mild increased signal in the periventricular WM on T2-weighted images, conventional MRI was normal. All children were attending normal mainstream schools. None had neurologic deficits or cerebral palsy on clinical examination.
Control subjects.
Thirteen control subjects (nine males, four females) aged 8.5–12.5 y (mean age, 10.11 y, SD = 1.18 y) were included for comparison with the preterm group.
There were no significant differences in gender (p = 0.501, Fisher's exact test), age [p = 0.605, Mann-Whitney U (M-U) test] and parental socioeconomic status (p = 0.079, M-U test) between the preterm group and the control group. Educational level was significantly lower in the preterm group compared with the control group (p = 0.045, M-U test).
MRI findings.
Mean WM volume and mean WM FA of the preterm group and the controls are summarized in Table 1, with stratification into male and female gender in Table 2. WM volume and FA was significantly lower in the preterm group compared with the control group (p = 0.014 and p < 0.001, respectively). Mean WM volume reduction was 7.35% and mean FA reduction was 4.95%. Males were found to have significantly lower FA compared with females in the premature group (p = 0.005). However, when the interaction of prematurity and gender on FA was tested using two-way analysis of variance, this was found to be not significant (p = 0.284). There was no significant difference in WM volume between males and females in the premature group.
IQ tests.
Of the 25 preterm children, 21 returned for IQ tests. The FSIQ was 106 ± 12.8 (mean ± SD). Verbal IQ and performance IQ were 109 ± 12.1 and 101 ± 13.9, respectively (mean ± SD).
Factors affecting cognitive function.
Using Spearman's univariate analysis, significant correlations were found between FSIQ and WM volume (r = 0.584, p = 0.005), FSIQ and BW (r = 0.485, p = 0.026), and FSIQ and GA (r = 0.474, p = 0.030). FA was not found to significantly affect FSIQ. On inspection of the scatterplot, we detected an outlier, but the correlation remained not significant even after removal of the outlier (p = 0.072). Subsequently, we performed multiple regression analysis after removal of the outlier.
Significant correlations were also found between GA and BW (r = 0.757, p < 0.001) and gender and FA (r = 0.592, p = 0.002). Gender difference was not shown to affect WM volume.
Multiple regression analysis found both WM volume and FA to be independent variables significantly affecting FSIQ after adjusting for GA, BW, and gender (r2 = 0.407, p = 0.021 and r2 = 0.496, p = 0.005, respectively) (Fig. 2A and B).
DISCUSSION
We studied quantitative parameters of WM damage by MRI using volumetry and DTI. In this cohort of neurologically normal low BW preterm children with minimal changes on conventional MRI findings, we found both mean WM volume and anisotropy to be reduced compared with term matched control subjects. In addition, both parameters significantly correlated with FSIQ, with WM FA having a stronger association than WM volume.
The mechanisms of perinatal brain injury in the preterm newborn are not fully understood. Although hypoxia-ischemia insults are a well-established cause of WMI, particularly in the preterm infant, recent studies demonstrate the role of inflammatory processes in perinatal preterm brain injury and the susceptibility of the developing oligodendrocytes toward ischemic inflammatory insults (33) and glutamate toxicity (34,35).
In a primate model of prematurity using baboons born prematurely, a marked predominance of cerebral WMI was observed in 50% of the prematurely born animals. The injury ranged from small patches of reactive astrocytosis to more extensive damage with activated microglia, small cystic lesions, and endothelial hypertrophy. The extent of WMI ranged from approximately 0.5% to 2.5% of total WM and occurred most frequently in the parietal and occipital lobes (36).
Our findings of reduced WM volume are in agreement with most studies of preterm children. These studies have found between 2.5% and 11% of total WM reduction in the preterm group (11–13). Up to 15% total WM reduction has been found in preterm infants with preexisting periventricular leukomalacia (37). Evaluation of the regional WM changes has shown diffuse alteration with areas of both deficit and excesses. Allin et al. (38) evaluated VLBW adults and found symmetrical WM excesses in the anterior part of the internal capsule, the insular cortex, and the arcuate fasciculus. WM deficits were seen symmetrically in the posterior part of the internal capsule and the superior, middle frontal, pre- and post-central gyri, and the optic radiation and unilaterally involving the left uncinate fasciculus. A recent preterm birth cohort of 50 adolescents showed diffuse regional WM volume decreases (18). Bilateral involvement of the superior and inferior longitudinal fasciculus was present. Other studies found a decrease in the corpus callosum (20,39) and the temporal lobe WM volume (40). Furthermore, regional WM volumes have been associated with neurodevelopmental outcome. Peterson et al. (21) showed that WM volumes of preterm infants in the sensorimotor and midtemporal regions, when assessed near term, correlated strongly with neurodevelopmental outcome at 18–20 mo of corrected age (Spearman's ρ > 0.9 for right sensorimotor and right midtemporal region, ρ = 0.83 for left sensorimotor region, ρ = 0.77 for left midtemporal region). Voxel-based morphometry analyses of the WM in a group of preterm, neurologically normal children at the age of 7.5–8 y revealed that regional WM volume in the parietal lobe was related to absolute IQ scores, whereas IQ decline over time was associated with regional WM volume in the frontal, temporal, and occipital regions (19).
In our cohort, we found a moderate but significant positive correlation of whole brain WM volume and FSIQ (r2 = 0.407, p = 0.021).
To date, only a few studies have evaluated the diffusion characteristics in WMI of prematurity at long-term follow up (28,29,41). It has been proposed that decreased WM anisotropy is related to poor myelination and axonal injury secondary to damage to the axonal-oligodendroglial unit (26). None have studied the relationship of the quantitative index, FA, with neurocognition in these cohorts. Significant correlations have been found between WM FA and IQ in both normal and diseased populations. Nagy et al. (42) found that the development of cognitive abilities in childhood is correlated with maturation of WM; specifically, working memory and reading abilities were found to correlate with increased FA in the superior and inferior left frontal lobe and the left temporal lobe respectively. Similar correlations have been found between cognitive function and chronic malignant phenylketonuria (43), Alzheimer's disease (44), ischemic leukoaraiosis (45), relapsing-remitting multiple sclerosis (46), and treatment-induced WMI in childhood cancer survivors (32). Recently, WM FA was found to correlate with FSIQ in the WM association areas of bilateral frontal and occipitoparietal regions in a cohort of normal children (47).
We found a trend of a more severe reduction in FA in premature males compared with females. Gender effect on FA in the premature cohort has not been described in the literature. However, preterm males appear to be particularly vulnerable to neurodevelopmental deficits compared with preterm females, with greater impairment in speech (48), learning, and academic achievement (49–51). Significant reduction in the total WM volume was found in preterm males compared with term males (11). Also, preterm adolescent boys were found to have a smaller mid-posterior callosal area compared with girls, and their verbal IQ and verbal fluency scores were positively associated with total mid-sagittal corpus callosum size and mid-posterior surface area (20). Thus, there is evidence that the male gender may be more susceptible to WMI than females.
Our cohort is limited by a relatively small sample size, and the study population was skewed toward children who all attend normal mainstream schools with the mildest form of learning problems.
With the decline in the incidence of overt pathologies such as periventricular leukomalacia and intraventricular hemorrhage, more sensitive MRI techniques with the use of quantitative indices may be required to detect and evaluate the subtle WM changes in the preterm brain.
Abbreviations
- BW:
-
birth weight
- DTI:
-
diffusion-tensor magnetic resonance imaging
- FA:
-
fractional anisotropy
- FSIQ:
-
full scale IQ
- GM:
-
gray matter
- M-U:
-
Mann-Whitney U test
- VLBW:
-
very low birth weight
- WM:
-
white matter
- WMI:
-
white matter injury
- 3D SPGR:
-
three-dimensional spoiled gradient recalled
References
Volpe JJ 2001 Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res 50: 553–562
Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N 1999 Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Dev Med Child Neurol 41: 826–833
Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH 2003 Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr 143: 163–170
Foulder-Hughes LA, Cooke RW 2003 Motor, cognitive, and behavioural disorders in children born very preterm. Dev Med Child Neurol 45: 97–103
Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ 2002 Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA 288: 728–737
McCarton CM, Brooks-Gunn J, Wallace IF, Bauer CR, Bennett FC, Bernbaum JC, Broyles RS, Casey PH, McCormick MC, Scott DT, Tyson J, Tonascia J, Meinert CL 1997 Results at age 8 years of early intervention for low-birth-weight premature infants. The Infant Health and Development Program. JAMA 277: 126–132
Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD 2000 Reduced development of cerebral cortex in extremely preterm infants. Lancet 356: 1162–1163
Vasileiadis GT, Gelman N, Han VK, Williams LA, Mann R, Bureau Y, Thompson RT 2004 Uncomplicated intraventricular hemorrhage is followed by reduced cortical volume at near-term age. Pediatrics 114: e367–e372
Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, Groenendaal F, de Vries LS, Huppi PS 2005 Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 116: 1–7
Martinussen M, Fischl B, Larsson HB, Skranes J, Kulseng S, Vangberg TR, Vik T, Brubakk AM, Haraldseth O, Dale AM 2005 Cerebral cortex thickness in 15-year-old adolescents with low birth weight measured by an automated MRI-based method. Brain 128: 2588–2596
Reiss AL, Kesler SR, Vohr B, Duncan CC, Katz KH, Pajot S, Schneider KC, Makuch RW, Ment LR 2004 Sex differences in cerebral volumes of 8-year-olds born preterm. J Pediatr 145: 242–249
Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM 2002 Adolescents who were born very preterm have decreased brain volumes. Brain 125: 1616–1623
Inder TE, Warfield SK, Wang H, Huppi PS, Volpe JJ 2005 Abnormal cerebral structure is present at term in premature infants. Pediatrics 115: 286–294
Peterson BS, Vohr B, Staib LH, Cannistraci CJ, Dolberg A, Schneider KC, Katz KH, Westerveld M, Sparrow S, Anderson AW, Duncan CC, Makuch RW, Gore JC, Ment LR 2000 Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA 284: 1939–1947
Abernethy LJ, Palaniappan M, Cooke RW 2002 Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Arch Dis Child 87: 279–283
Abernethy LJ, Cooke RW, Foulder-Hughes L 2004 Caudate and hippocampal volumes, intelligence, and motor impairment in 7-year-old children who were born preterm. Pediatr Res 55: 884–893
Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ 2003 Defining the nature of the cerebral abnormalities in the premature infant: a qualitative magnetic resonance imaging study. J Pediatr 143: 171–179
Gimenez M, Junque C, Narberhaus A, Bargallo N, Botet F, Mercader JM 2006 White matter volume and concentration reductions in adolescents with history of very preterm birth: a voxel-based morphometry study. Neuroimage 32: 1485–1498
Isaacs EB, Edmonds CJ, Chong WK, Lucas A, Morley R, Gadian DG 2004 Brain morphometry and IQ measurements in preterm children. Brain 127: 2595–2607
Nosarti C, Rushe TM, Woodruff PW, Stewart AL, Rifkin L, Murray RM 2004 Corpus callosum size and very preterm birth: relationship to neuropsychological outcome. Brain 127: 2080–2089
Peterson BS, Anderson AW, Ehrenkranz R, Staib LH, Tageldin M, Colson E, Gore JC, Duncan CC, Makuch R, Ment LR 2003 Regional brain volumes and their later neurodevelopmental correlates in term and preterm infants. Pediatrics 111: 939–948
Beaulieu C 2002 The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed 15: 435–455
Counsell SJ, Boardman JP 2005 Differential brain growth in the infant born preterm: current knowledge and future developments from brain imaging. Semin Fetal Neonatal Med 10: 403–410
Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB 2004 Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage 22: 1302–1314
Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ 1998 Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44: 584–590
Huppi PS, Murphy B, Maier SE, Zientara GP, Inder TE, Barnes PD, Kikinis R, Jolesz FA, Volpe JJ 2001 Microstructural brain development after perinatal cerebral white matter injury assessed by diffusion tensor magnetic resonance imaging. Pediatrics 107: 455–460
Miller SP, Vigneron DB, Henry RG, Bohland MA, Ceppi-Cozzio C, Hoffman C, Newton N, Partridge JC, Ferriero DM, Barkovich AJ 2002 Serial quantitative diffusion tensor MRI of the premature brain: development in newborns with and without injury. J Magn Reson Imaging 16: 621–632
Nagy Z, Westerberg H, Skare S, Andersson JL, Lilja A, Flodmark O, Fernell E, Holmberg K, Bohm B, Forssberg H, Lagercrantz H, Klingberg T 2003 Preterm children have disturbances of white matter at 11 years of age as shown by diffusion tensor imaging. Pediatr Res 54: 672–679
Vangberg TR, Skranes J, Dale AM, Martinussen M, Brubakk AM, Haraldseth O 2006 Changes in white matter diffusion anisotropy in adolescents born prematurely. Neuroimage 32: 1538–1548
Hong Kong: Education and Manpower Bureau 1981 Hong Kong Wechsler Intelligence Scale for children. Translated and adapted from Wechsler Intelligence Scale for Children by permission of New York: The Psychological Corporation
Wilke M, Schmithorst VJ, Holland SK 2002 Assessment of spatial normalization of whole-brain magnetic resonance images in children. Hum Brain Mapp 17: 48–60
Khong PL, Leung LH, Fung AS, Fong DY, Qiu D, Kwong DL, Ooi GC, McAlanon G, Cao G, Chan GC 2006 White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol 24: 884–890
du Plessis AJ, Volpe JJ 2002 Perinatal brain injury in the preterm and term newborn. Curr Opin Neurol 15: 151–157
Yoshioka A, Bacskai B, Pleasure D 1996 Pathophysiology of oligodendroglial excitotoxicity. J Neurosci Res 46: 427–437
Oka A, Belliveau MJ, Rosenberg PA, Volpe JJ 1993 Vulnerability of oligodendroglia to glutamate: pharmacology, mechanisms, and prevention. J Neurosci 13: 1441–1453
Inder T, Neil J, Yoder B, Rees S 2005 Patterns of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Child Neurol 20: 965–967
Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ 1999 Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol 46: 755–760
Allin M, Henderson M, Suckling J, Nosarti C, Rushe T, Fearon P, Stewart AL, Bullmore ET, Rifkin L, Murray R 2004 Effects of very low birthweight on brain structure in adulthood. Dev Med Child Neurol 46: 46–53
Fearon P, O'Connell P, Frangou S, Aquino P, Nosarti C, Allin M, Taylor M, Stewart A, Rifkin L, Murray R 2004 Brain volumes in adult survivors of very low birth weight: a sibling-controlled study. Pediatrics 114: 367–371
Kesler SR, Ment LR, Vohr B, Pajot SK, Schneider KC, Katz KH, Ebbitt TB, Duncan CC, Makuch RW, Reiss AL 2004 Volumetric analysis of regional cerebral development in preterm children. Pediatr Neurol 31: 318–325
Counsell SJ, Shen Y, Boardman JP, Larkman DJ, Kapellou O, Ward P, Allsop JM, Cowan FM, Hajnal JV, Edwards AD, Rutherford MA 2006 Axial and radial diffusivity in preterm infants who have diffuse white matter changes on magnetic resonance imaging at term-equivalent age. Pediatrics 117: 376–386
Nagy Z, Westerberg H, Klingberg T 2004 Maturation of white matter is associated with the development of cognitive functions during childhood. J Cogn Neurosci 16: 1227–1233
Peng SS, Tseng WY, Chien YH, Hwu WL, Liu HM 2004 Diffusion tensor images in children with early-treated, chronic, malignant phenylketonuric: correlation with intelligence assessment. AJNR Am J Neuroradiol 25: 1569–1574
Kantarci K, Jack CR Jr, Xu YC, Campeau NG, O'Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC 2001 Mild cognitive impairment and Alzheimer disease: regional diffusivity of water. Radiology 219: 101–107
O'sullivan M, Morris RG, Huckstep B, Jones DK, Williams SC, Markus HS 2004 Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J Neurol Neurosurg Psychiatry 75: 441–447
Rovaris M, Iannucci G, Falautano M, Possa F, Martinelli V, Comi G, Filippi M 2002 Cognitive dysfunction in patients with mildly disabling relapsing-remitting multiple sclerosis: an exploratory study with diffusion tensor MR imaging. J Neurol Sci 195: 103–109
Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK 2005 Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp 26: 139–147
Ment LR, Peterson BS, Meltzer JA, Vohr B, Allan W, Katz KH, Lacadie C, Schneider KC, Duncan CC, Makuch RW, Constable RT 2006 A functional magnetic resonance imaging study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics 118: 961–970
Hindmarsh GJ, O'Callaghan MJ, Mohay HA, Rogers YM 2000 Gender differences in cognitive abilities at 2 years in ELBW infants. Extremely low birth weight. Early Hum Dev 60: 115–122
Johnson EO, Breslau N 2000 Increased risk of learning disabilities in low birth weight boys at age 11 years. Biol Psychiatry 47: 490–500
Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N 2002 Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med 346: 149–157
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by the University of Hong Kong Committee on Research and Conference grants (CRCG).
Rights and permissions
About this article
Cite this article
Yung, A., Poon, G., Qiu, DQ. et al. White Matter Volume and Anisotropy in Preterm Children: A Pilot Study of Neurocognitive Correlates. Pediatr Res 61, 732–736 (2007). https://doi.org/10.1203/pdr.0b013e31805365db
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/pdr.0b013e31805365db
This article is cited by
-
Functional and structural connectivity of the brain in very preterm babies: relationship with gestational age and body and brain growth
Pediatric Radiology (2019)
-
Neuroimaging in former preterm children who received erythropoiesis stimulating agents
Pediatric Research (2017)
-
Diffusion magnetic resonance imaging in preterm brain injury
Neuroradiology (2013)
-
Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children
Pediatric Radiology (2011)
-
Neuro-developmental outcome at 18 months in premature infants with diffuse excessive high signal intensity on MR imaging of the brain
Pediatric Radiology (2011)