Abstract
The precise role of CO2 in cerebral oxygenation is not as well defined as O2, especially in the immature brain. In the ovine fetus, we tested the hypotheses that arterial Pco2 (Paco2) plays a critical role not only in the regulation of cerebral blood flow but also in the regulation of cerebral tissue oxygenation. By use of a fluorescent O2 probe with a laser Doppler flowmeter and the placement of sagittal sinus catheter in six near-term fetal sheep, we measured values of cortical tissue O2 tension (tPo2), sagittal sinus oxyhemoglobin saturation ([HbO2]), and laser Doppler cerebral blood flow (LD-CBF) in response to 20 min hypercapnia induced by having the ewe breathe CO2. In response to moderate to severe hypercapnia, LD-CBF increased above baseline in a curvilinear fashion, cortical tPo2 increased linearly (1 torr per 3.2 torr Paco2), and sagittal sinus [HbO2] increased significantly in a curvilinear manner. Hypercapnia favored cerebral tissue oxygenation of the fetal brain; and cortical tPo2 and sagittal sinus [HbO2] complement or support one another as indices of cerebral oxygenation under hypercapnic conditions.
Similar content being viewed by others
Main
In the developing fetus and newborn infant, as well as the adult, the maintenance of optimal cerebral oxygenation is of critical importance, as the brain is highly dependent on a continuous and adequate O2 supply to maintain structural and functional integrity. Cerebral tissue oxygenation is maintained and carefully regulated by the balance of several factors. These include CBF, arterial O2 partial pressure (Pao2) and content, cerebral metabolic rate for O2 (CMRO2), and the relative position of the Hb-oxygen dissociation curve (1,2). Arterial CO2 tension (Paco2) long has been recognized as playing a major role in CBF regulation of the fetus (3) as well as the adult (4,5). Thus, it is reasonable to anticipate that CO2, as well as O2, plays a significant role in cerebral tissue oxygenation.
Variation of Paco2 commonly occurs in the management of the fetus and newborn during the perinatal period. During labor, fetal hypercapnia with respiratory acidosis, in part resulting from transient compression of the umbilical cord, is not uncommonly seen (6). In addition, vaginal delivery itself may be associated with respiratory acidosis and hypoxia (7). Alternatively, with moderate to severe maternal hyperventilation, particularly during the later stages of labor, fetal hypocapnia with respiratory alkalosis may develop (8,9). In addition, among critically ill newborn infants, significant changes in Paco2 can present problems. Under some circumstances, hypocapnia is believed to be of value to prevent an excessive increase in CBF. In other instances, “permissive” hypercapnia is practiced to optimize CBF, to reduce the risk of periventricular leukomalacia, and to minimize ventilator-associated lung injury (10). Nonetheless, despite the critical importance of these issues, and knowledge that cerebrovascular Paco2 reactivity differs dramatically between the immature and mature organism (11), the role of CO2 in cerebral tissue oxygenation has received relatively little attention, especially in the immature brain. To our knowledge, no studies have addressed this issue in the fetus.
By the use of cortical tPo2 and ss [HbO2] values, we tested the hypothesis that hypercapnia favors cerebral tissue oxygenation in the fetal brain. In addition, we explored the following questions. What is the dose-response relationship of fetal LD-CBF to Paco2 and to sagittal sinus Pco2? What are the dose-response relationships of both cortical tPo2 and ss [HbO2] to Paco2 values? What is the relationship of cortical tPo2 to ss [HbO2] under various levels of hypercapnia? In this study, we used chronically catheterized near-term fetal sheep, as several anesthetic agents have been reported to affect cerebral oxygenation (12–14).
MATERIALS AND METHODS
Experimental animals and instrumentation.
For these studies, we used six pregnant Western ewes and their singleton fetuses obtained from Nebeker Ranch (Lancaster, CA). All surgical and experimental procedures were performed within the regulations of the Animal Welfare Act, the National Institutes of Health Guide for the Care and Use of Laboratory Animals, “The Guiding Principles in the Care and Use of Animals” approved by the Council of the American Physiologic Society, and the Animal Care and Use Committee of Loma Linda University.
Pregnant ewes and their fetuses were instrumented at 122 ± 3 d of gestation (term 145 d), as we have described in a previous report (15). Briefly, anesthesia was maintained throughout the surgical procedure with mechanical ventilation of 1.0% halothane in oxygen. All surgical procedures were carried out under aseptic conditions. Following anesthesia, the maternal abdominal wall and uterus were incised and the fetal head and forelimbs delivered. In each forelimb, we inserted a polyvinyl catheter (2.3 mm o.d.) into the brachial artery and advanced it into the aortic arch for arterial blood sampling and recording of blood pressure and heart rate. We also placed a catheter into the brachial vein, and advanced it into the superior vena cava.
We then incised the scalp rostral to the coronal suture, exposing the right and left parietal bones. We drilled a 1.5-mm burr hole on the right side 5 mm lateral to the sagittal suture and 15 mm caudal to the coronal suture. We inserted the tip of the composite tPo2–laser Doppler flow (LDF) probe with thermocouple (Oxford Optronix, Ltd., Oxford, UK) into the cortex of the parasagittal parietal lobe to a depth of 5 mm below the dura mater, and fixed this to the skull with a custom-made probe holder. We repeated the same steps on the left side, and then closed the scalp incision. We also placed a polyvinyl catheter (2.3 mm o.d.) 1.5 cm into the sagittal sinus, and this catheter enabled sampling of mixed venous blood from the anterior brain, including the tissue containing the tPo2-LDF probe. Next, we placed a polyvinyl catheter (3.5 mm o.d.) into the amniotic fluid for measurement of amniotic fluid pressure and administration of antibiotics. The uterine wall was closed in layers, and catheters and probe connections were exteriorized to the ewe's left flank and stored in a pouch attached to the maternal skin. Lastly, in the ewe's right femoral artery and vein we inserted Tygon polyvinyl catheters. Postoperatively, the ewe was given 900,000 U penicillin intramuscularly for 3 d, and the fetus was given cefotaxime (50 mg/d, i.v.). We also administered ampicillin (500 mg) and gentamicin (40 mg) into the amniotic fluid daily until the experiments were completed. We monitored sheep well being and arterial blood gases daily for 4 to 5 d of postoperative recovery before commencing the experiments.
Experimental design.
The protocol was designed to measure cortical tPo2, ss [HbO2], and LD-CBF during a 40-min normoxic control period, followed by 20 min hypercapnia. We induced hypercapnia by having the ewe breathe CO2 in air to increase fetal Paco2 values. This was administered by passing an air plus CO2 gas mixture at 30 L·min–1 through an opaque plastic bag over the ewe's head. For study of Paco2 dose-response, the inspired CO2 was increased gradually so that over the 20-min period fetal Paco2 increased to 70 torr (see Fig. 1A). We collected fetal arterial and sagittal sinus blood samples (0.3 mL each) every 20 min throughout the control period, and every 5 min during hypercapnia, and analyzed for blood gases (ABL3, Radiometer, Copenhagen, Denmark). We corrected blood gas values to the fetal body temperature (16). We also measured spectrophotometrically Hb concentration [Hgb] and oxyhemoglobin saturation [HbO2] (OSM2 Hemoximeter, Radiometer), and calculated O2 content as the product of [HbO2] × [Hgb] × 1.34. We calculated relative cerebral O2 delivery (LD-CBF × arterial O2 content) and cerebral fractional O2 extraction, i.e. O2 consumed as a fraction of that delivered (cerebral metabolic rate for O2/cerebral O2 delivery, which reduces to 1 – venous O2 content/arterial O2 content) (2). We also calculated CMRO2 as the product of relative LD-CBF × arterial to sagittal sinus O2 content difference. In addition, we measured plasma glucose and lactate concentrations (Model 2700, Select Biochemistry Analyzer, v. 2.50D; YSI Inc., Yellow Springs, OH).
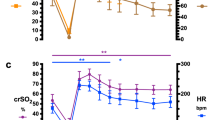
Response of near-term fetus to hypercapnia in which Paco2 was gradually increased (A) during the experimental protocol, with a 40-min control period, followed by 20 min hypercapnia. Hypercapnia was associated with increases in LD-CBF (B), cortical tPo2 (C), and ss [HbO2] (D). Data points represent means ± SEM.
Data acquisition and statistical analyses.
We measured continuously cortical tPo2 and LD-CBF, MABP, and FHR. The data used for Figures 2–4 and the tables were obtained at the time of blood sampling. Analog outputs were digitized (sampling rate 100 Hz) and stored using an analogue to digital converter and data acquisition software (Powerlab 16/SP, and Chart v 4, ADInstruments, Colorado Springs, CO). We recorded tPo2 and LD-CBF signals from both right and left hemispheres of the brain; these two results were averaged to provide mean values. Because LD-CBF provides a relative, not absolute, measure of LD-CBF and cerebral O2 delivery for each animal, we calculated these as a percentage of the mean values during the baseline control period. For tables, for each animal, all control data were pooled to obtain a single value. During the hypercapnic period, we pooled data from the first 10 min of mild to moderate hypercapnia and also from the last 10 min of more severe hypercapnia.
Relative LD-CBF and cerebral O2 delivery in response to hypercapnia in the near-term fetus. (A) Relation of fetal LD-CBF (% baseline control) to Paco2 (torr) (LD-CBF = –71.1 + 5.28 Paco2N 0.03 Paco22; r2 = 0.77, p < 0.0001). (B) Relation of fetal LD-CBF (% control) to ss Pco2 (torr) (LD-CBF = –111.4 + 6.10 ss Pco2N 0.04 ss Pco22 r2 = 0.62, p < 0.001). (C) Relation of fetal cerebral O2 delivery (% baseline control) to Paco2 (torr) (cerebral O2 delivery = 37.9 + 1.43 Paco2, r2 = 0.63, p < 0.0001). Shown are regression lines with 95% confidence limits.
Relation of ss [HbO2] to cortical tPo2 under two different levels of Paco2. Solid triangles show the relation with Paco2 < 60 torr (ss [HbO2] = 32.1 + 1.18 cortical tPo2, r2 = 0.41, p < 0.0001). Open triangles show this relation with Paco2> 60 torr (ss [HbO2] = 52.3 + 0.02 cortical tPo2, r2 = 0.0002, p = 0.95). Shown are regression lines for each variable.
Results were expressed as means ± SEM and analyzed using one-way ANOVA with repeated measures, followed by Newman-Keuls post hoc test. These and the linear regressions were performed using GraphPad Prism (GraphPad Software, San Diego, CA). A value of p < 0.05 was considered statistically significant.
RESULTS
To determine the dose-response relationship of several variables to fetal Paco2, we increased CO2 levels over a 20-min period. Figure 1A depicts the experimental protocol. Following a 40-min control period, we induced hypercapnia, gradually increasing fetal Paco2 over a 20-min period. In response to hypercapnia, the mean fetal Paco2 increased to 68 ± 3 torr (Table 1). In addition, fetal laser Doppler cerebral blood flow increased 32 ± 2% above control (Fig. 1B), and cortical tissue Po2 increased from 8 ± 1 torr to 18 ± 2 torr (see Fig. 1C and Table 1). In addition, in response to hypercapnia, sagittal sinus oxyhemoglobin saturation increased, plateauing after 10 min at 52 ± 1% (Fig. 1D and Table 2). Following hypercapnia, fetal LD-CBF and other variables returned to control values within 10 to 15 min, and remained stable.
An important question regards the extent to which fetal LD-CBF increases as a function of Paco2. Figure 2A illustrates this dose-response relationship of fetal LD-CBF to Paco2. As is evident, LD-CBF increased in a curvilinear manner as Paco2 increased, tending to plateau at Paco2>70 torr. One might ask, is LD-CBF more closely related to ss Pco2 than to the arterial value? Figure 2B presents this relationship; although Pco2 values on the abscissa differ from those in Figure 2A, the curvilinear relationship is similar. Additionally, the question arises as to the relation of fetal cerebral O2 delivery to Paco2. Figure 2C shows the relative cerebral O2 delivery [the product of LD-CBF (% baseline) and the arterial O2 content; see Table 1]. As seen in Figure 2 C, with the hypercapnic-associated increase in LD-CBF, cerebral O2 delivery also increased significantly as a function of elevated Paco2. The regression equation for this relationship is given in the figure legend.
One also may inquire as to the relation of fetal cortical Po2 to Paco2, and the extent to which this is similar to its relation to ss Pco2. Figure 3A illustrates the dose-response relationships of fetal cortical tPo2 to Paco2, cortical tPo2 increasing linearly as a function of elevated Paco2 from 8 ± 1 torr to 18 ± 2 torr (1 torr increase in cortical tPo2 per 3.2 torr increase in Paco2). There was no apparent threshold within this range, and the regression equation is given in the figure legend. As seen in Figure 3B, cortical tPo2 also increased linearly in response to elevated ss Pco2. In addition, as seen in Figure 3C, ss [HbO2] increased in a nonlinear manner from 41 ± 1% at Paco2 of 46 ± 1 torr to 52 ± 1% at Paco2 value of 68 ± 3 torr (see Tables 1 and 2). This relationship of ss [HbO2] to Paco2 was fairly linear until Paco2 60 torr, plateauing above this level (Fig. 3C). Additionally, ss [HbO2] increased in a similar curvilinear manner as a function of ss Pco2 (Fig. 3D).
Response of cortical tPo2 and ss [HbO2] to hypercapnia in the near-term fetus. (A) Relation of cortical tPo2 (torr) to Paco2 (torr) (cortical tPo2 = –4.1 + 0.31 Paco2, r2 = 0.59, p < 0.0001). (B) Relation of cortical tPo2 (torr) to ss Pco2 (torr) (cortical tPo2 = 4.9 + 0.29 ss Pco2, r2 = 0.46, p < 0.001). (C) Relation of ss [HbO2] to Paco2 (torr) (ss [HbO2] = – 33 + 2.36 Paco2N 0.016 Paco22, r2 = 0.52, p < 0.0001). (D) Relation of ss [HbO2] to ss Pco2 (ss [Hb O2] (torr) = –50.2 + 2.67 ss Pco2N0.017 ss Pco22, r2 = 0.49, p < 0.001). Shown are regression lines (with 95% confidence limits for A and B).
Figure 4 shows the relationship of cortical tPo2 to ss [HbO2] values under two levels of hypercapnia (less than and greater than 60 torr). Correlation of these values was fairly linear until Paco2 60 torr (r2 = 0.41, p < 0.0001, shown as closed triangles). This correlation was lost, however, at Paco2>60 torr (r2 = 0.0002, p = 0.95, shown as open triangles).
In response to hypercapnia, the fetal arterial to sagittal sinus O2 content difference decreased 53% from 1.5 ± 0.1 to 0.7 ± 0.2 mM (Table 2). The fractional O2 extraction also decreased 49% (Table 2). In addition, at maximal hypercapnia (Paco2 = 68 ± 3 torr) despite the 32% increase in LD-CBF, because of the 53% decrease in arterial to sagittal sinus O2 difference, the cerebral metabolic rate for O2 decreased 34% (Table 2). The FHR, MABP, and the plasma lactate and glucose concentrations showed no significant change with hypercapnia (Table 1).
DISCUSSION
The relation of cerebral tissue oxygenation to hypercapnia is not well described, especially in the immature brain. The present study demonstrates for the first time the significant increase in cortical tPo2 (Fig. 3, A and B) and ss [HbO2] (Fig. 3, C and D) in response to hypercapnia in the near-term fetus.
In the fetal brain, the present study demonstrated a significant hypercapnic-induced increase in LD-CBF (Fig. 1B and Fig. 2, A and B) and O2 delivery (Fig. 2C). These would appear to be the major factors for the improvement of cerebral tissue oxygenation in response to hypercapnia. In humans and most other mammals, CO2 is a powerful vasodilator constituting a predominant influence in the regulation of CBF. The CO2 vasodilatory effect has been considered to result from hypercapnia-induced cerebral extracellular acidosis (4,5). In addition, in the newborn piglet, CO2 affects the vascular endothelium (17). This may account for the present results, demonstrating no significant difference in the LD-CBF responses as a function of ss Pco2, as opposed to arterial Pco2 (Fig. 2, A and B). In immature cerebral blood vessels, the CBF responses to increased Paco2 appear to be not as well developed as in the adult (11). It thus seems reasonable to anticipate that the cerebral tissue oxygenation response to Paco2 in the fetus should be less than that of the adult. In accordance with this idea, we conducted a similar experiment in unanesthetized adult sheep and found that the cortical tPo2 response to Paco2 was three times as great (1 torr per 1 torr Paco2) than that of the fetus (1 torr per 3.2 torr Paco2) (unpublished data). This result in adult sheep also was consistent with that reported in anesthetized adult rats (18).
In this study, we used two methods used clinically (cerebral tissue O2 tension and cerebral venous oxyhemoglobin saturation) to estimate cerebral oxygenation. Measurement of cerebral tissue O2 tension is a direct and accurate measurement of tissue Po2, however, it represents oxygenation of a fairly discrete region of the brain (parasagittal parietal cortex, in this study). Another possible limitation of this method is that the placement of the probe may result in local tissue trauma and scar formation, leading to an inaccurate value. Previously, in a similar preparation we carefully examined the question of the extent to which CBF was altered by the presence of the probe (19). In our microsphere measurements in 27 cubes 4 mm on edge surrounding the probe, probe placement had not altered CBF appreciably, examined 5 d after surgery (19). Based on this, it is reasonable to anticipate that cortical tPo2 also may not be altered significantly by tissue trauma. Also, the sagittal sinus drains blood from the anterior one-third of the fetal lamb brain (20), and its oxyhemoglobin saturation is considered to represent global cerebral oxygenation. Nonetheless, this value is an indirect estimation of cerebral (chiefly cortex and partly white matter; in this study) oxygenation, and small contributions by extracerebral contamination cannot be excluded. These issues may account for the different shapes of dose-response curves of cortical tPo2 and ss [HbO2] to PaCO2 (Fig. 3, A and C) and to ss Pco2 (Fig. 3, B and D), as observed in the present study.
The Paco2/pH-associated shift to the right in the oxyhemoglobin dissociation curve, e.g. Bohr shift enhances the release of O2 from oxyhemoglobin to the brain tissue. Theoretically, it is reasonable to anticipate that a Bohr shift favors an increase in cortical tPo2 value, while decreasing ss [HbO2], and that the clinical value of venous oxyhemoglobin saturation may be limited under the alternation of Hb oxygen affinity caused by hypothermia, hyperthermia, alkalosis, acidosis, or other factors. This also may be one of the reasons for the different shape of the dose-response curves of cortical tPo2 and ss [HbO2] to Paco2 and to ss Pco2. This also may explain a mismatch of cerebral tissue Po2 and jugular venous [HbO2] values with hyperventilation (21), and the desaturation of jugular venous blood during rewarming following hypothermic cardiopulmonary bypass (22). One also might argue that the observed increase in fetal Pao2 (from 22 ± 1 to 26 ± 1 torr) contributed to improvement in cerebral tissue oxygenation. However, it is not likely as neither arterial [HbO2] nor O2 content increased significantly (Table 1), probably secondary to the rightward shift of the oxyhemoglobin dissociation curve.
The CMRO2 is also one of the important factors in determining cerebral tissue oxygenation. If we use the relative increase in LD-CBF and Fick principle as an equation for calculation, in this study relative CMRO2 decreased 34% in response to hypercapnia. This finding also agrees with several studies in adult animals that demonstrated significantly reduced CMRO2 in response to hypercapnia (23,24). One must be cautious here, however. If, in fact, the hypercapnic-induced increase in cerebral blood flow was greater than that we recorded with laser-Doppler, CMRO2 would not decrease as much, or perhaps even remain constant. One study also demonstrated that in very low birth weight newborns (birth weight <1500 g, gestation <30 wk), elevated Paco2 levels were associated with suppression of electroencephalographic activity (25). Nonetheless, this issue is controversial. As we (19) and others (26) have shown, LD-CBF may underestimate the CBF increase, compared with the microsphere technique. Also, one group has reported no correlation between CMRO2 and Paco2 in response to a similar level of hypercapnia in fetal and newborn sheep using the microsphere technique (11). Thus, data on the effect of hypercapnia on fetal CMRO2 requires further study.
For the near-term fetus, we present the first dose-response data on the relation of cortical tPo2 and ss [HbO2] values to both arterial and ss Pco2 with various levels of hypercapnia. We also present the relation of cortical tPo2 to ss [HbO2] and Pco2 under these conditions. In the fetal brain, Paco2 would appear to be a critical determinant of cerebral tissue oxygenation, as well as CBF. Cortical tPo2 increased linearly with Paco2 (Fig. 3A) and ss Pco2 (Fig. 3B), probably reflecting the increase in CBF (Fig. 2A), cerebral O2 delivery (Fig. 2C), and the Paco2/pH-associated shift to the right in the oxyhemoglobin dissociation curve. Sagittal sinus [HbO2] also increased in response to hypercapnia, although this relation to Paco2 and to ss Pco2 tended to plateau when the Pco2 exceeded 60 torr (Fig. 3, C and D). In addition, despite a significant correlation of cortical tPo2 and ss [HbO2] with mild hypercapnia, this relation was lost in the presence of more severe hypercapnia (Fig. 4).
In a clinical study, fetal respiratory acidosis with no metabolic component has been reported not to be associated with newborn complications, including neonatal encephalopathy (27). In addition, in the management of the premature infant “permissive hypercapnia” with mild respiratory acidosis has been anticipated to reduce the risk of periventricular leukomalacia (10,28). In contrast, accumulating evidence suggests the adverse neurologic consequences of hypocapnia, explained in part by cerebral vasoconstriction and increasing Hb O2 affinity (21,29,30). In accordance with these findings, in the immature rat Vannucci and co-workers (31) have shown that hypercapnic (Paco2 = 54 torr) cerebral hypoxia-ischemia was more neuroprotective than normocapnic hypoxia-ischemia, and hypocapnic hypoxia-ischemia was associated with more severe brain damage than that which is normocapnic. Our findings in the present study of hypercapnic-induced increase in cortical tissue Po2 and ss [HbO2] support these concepts, suggesting a protective role of hypercapnia on the CNS. As a cautionary note, in the newborn piglet one study suggested adverse effects, including altered cerebral cortex nuclear enzyme activity and protein expression, of severe hypercapnia (Paco2 = 65–80 torr) (32). In turn, the decrease in fractional O2 extraction and CMRO2 seen in the present study may be a basis of these changes. Thus, the present study suggests that the effects of hypercapnia on brain oxygenation may be quite complex. Quite obviously, further studies will be required to determine the risks-benefits of hypercapnia in the developing brain, and the proper management of the newborn infant and fetus, in terms of optimal maintenance of cerebral tissue oxygenation.
Abbreviations
- CBF:
-
cerebral blood flow
- CMRO2:
-
cerebral metabolic rate for O2
- FHR:
-
fetal heart rate
- LD-CBF:
-
laser Doppler cerebral blood flow
- MABP:
-
mean arterial blood pressure
- ss [HbO2]:
-
sagittal sinus oxyhemoglobin saturation
- tPO2:
-
cortical tissue O2 tension
References
Kreuzer F 1982 Oxygen supply to tissues: the Krogh model and its assumptions. Experientia 38: 1415–1426
Jones MD Jr, Traystman RJ 1984 Cerebral oxygenation of the fetus, newborn, and adult. Semin Perinatol 8: 205–216
Ashwal S, Dale PS, Longo LD 1984 Regional cerebral blood flow: studies in fetal lamb during hypoxia, hypercapnia, acidosis, and hypotension. Pediatr Res 18: 1309–1316
Kontos HA, Raper J, Patterson JL Jr 1977 Analysis of vasoactivity of local pH, PCO2 and bicarbonate on pial vessels. Stroke 8: 358–360
Madden JA 1993 The effect of carbon dioxide on cerebral arteries. Pharmacol Ther 59: 229–250
Cunningham FG, Lenko KJ, Bloom SH, Hauth JC, Gilstrap L 3rd, Wenstrom KD 2005 The newborn infant. In: Cunningham FG, Lenko KJ, Bloom SH, Hauth JC, Gilstrap L 3rd, Wenstrom KD (eds) Williams Obstetrics. McGraw-Hill, New York, pp 633–647
Gregg AR, Weiner CP 1993 “Normal” umbilical arterial and venous acid-base and blood gas values. Clin Obstet Gynecol 36: 24–32
Huch R 1986 Maternal hyperventilation and the fetus. J Perinat Med 14: 3–18
Longo LD 1987 Respiratory gas exchange in the placenta. In: Fishman AP, Farhi LE, Tenney SM (eds) Handbook of Physiology, Section 3: The Respiratory System, Vol. IV, Gas Exchange. American Physiological Society, Washington DC, pp 35l–40l
Thome UH, Carlo WA 2002 Permissive hypercapnia. Semin Neonatol 7: 409–419
Rosenberg AA, Jones MD Jr, Traystman RJ, Simmons MA, Molteni RA 1982 Response of cerebral blood flow to changes in PCO2 in fetal, newborn, and adult sheep. Am J Physiol 242: H862–H866
Hoffman WE, Charbel FT, Edelman G, Misra M, Ausman JI 1998 Comparison of the effect of etomidate and desflurane on brain tissue gases and pH during prolonged middle cerebral artery occlusion. Anesthesiology 88: 1188–1191
Hoffman WE, Edelman G 2000 Enhancement of brain tissue oxygenation during high dose isoflurane anesthesia in the dog. J Neurosurg Anesthesiol 12: 95–98
McClaine RJ, Uemura K, de la Fuente SG, Manson RJ, Booth JV, White WD, Campbell KA, McClaine DJ, Benni PB, Eubanks WS, Reynolds JD 2005 General anesthesia improves fetal cerebral oxygenation without evidence of subsequent neuronal injury. J Cereb Blood Flow Metab 25: 1060–1069
Tomimatsu T, Pereyra Peña JL, Hatran DP, Longo LD 2006 Maternal oxygen administration and fetal cerebral oxygenation: studies on near-term fetal lambs at both low and high altitude. Am J Obstet Gynecol 195: 535–541
Lotgering FK, Gilbert RD, Longo LD 1983 Exercise responses in pregnant sheep: Blood gases, temperatures, and fetal cardiovascular system. J Appl Physiol 55: 842–850
Leffler CW, Mirro R, Shanklin DR, Armstead WM, Shibata M 1994 Light/dye microvascular injury selectively eliminates hypercapnia-induced pial arteriolar dilation in newborn pigs. Am J Physiol 266: H623–H630
Hare GM, Kavanagh BP, Mazer CD, Hum KM, Kim SY, Coackley C, Barr A, Baker AJ 2003 Hypercapnia increases cerebral tissue oxygen tension in anesthetized rats. Can J Anaesth 50: 1061–1068
Bishai JM, Blood AB, Hunter CJ, Longo LD, Power GG 2003 Fetal lamb cerebral blood flow (CBF) and oxygen tensions during hypoxia: a comparison of laser Doppler and microsphere measurements of CBF. J Physiol 546( Pt 3): 869–878
Grant DA, Franzini C, Wild J, Walker AM 1995 Continuous measurement of blood flow in the superior sagittal sinus of the lamb. Am J Physiol 269: R274–R279
Imberti R, Bellinzona G, Langer M 2002 Cerebral tissue PO2 and SjvO2 changes during moderate hyperventilation in patients with severe traumatic brain injury. J Neurosurg 96: 97–102
Souter MJ, Andrews PD, Alston RP 1998 Jugular venous desaturation following cardiac surgery. Br J Anaesth 81: 239–241
Kliefoth AB, Grubb RL Jr, Raichle ME 1979 Depression of cerebral oxygen utilization by hypercapnia in the rhesus monkey. J Neurochem 32: 661–663
Artru AA, Michenfelder JD 1980 Effects of hypercarbia on canine cerebral metabolism and blood flow with simultaneous direct and indirect measurement of blood flow. Anesthesiology 52: 466–469
Victor S, Appleton RE, Beirne M, Marson AG, Weindling AM 2005 Effect of carbon dioxide on background cerebral electrical activity and fractional oxygen extraction in very low birth weight infants just after birth. Pediatr Res 58: 579–585
Kirkeby OJ, Rise IR, Nordsletten L, Skjeldal S, Hall C, Risoe C 1995 Cerebral blood flow measured with intracerebral laser-Doppler flow probes and radioactive microspheres. J Appl Physiol 79: 1479–1486
Low JA, Panagiotopoulous C, Derrick EJ 1994 Newborn complications after intrapartum asphyxia with metabolic acidosis in the term infants. Am J Obstet Gynecol 170: 1081–1087
Wung JT, James LS, Kilchevsky E, James E 1985 Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics 76: 488–494
Wilson DF, Pastuszko A, DiGiacomo JE, Pawlowski M, Schneiderman R, Delivoria-Papadopoulos M 1991 Effect of hyperventilation on oxygenation of the brain cortex of newborn piglets. J Appl Physiol 70: 2691–2696
Laffey JG, Kavanagh BP Hypocapnia. N Engl J Med 2002 347: 43–53
Vannucci RC, Towfighi J, Heitjan DF, Brucklacher RM 1995 Carbon dioxide protects the perinatal brain from hypoxic-ischemic damage: an experimental study in the immature rat. Pediatrics 95: 868–874
Fritz KI, Zubrow A, Mishra OP, Delivoria-Papadopoulos M 2005 Hypercapnic-induced modifications of neuronal function in the cerebral cortex of newborn piglets. Pediatr Res 57: 299–304
Acknowledgements
The authors thank Douglas P. Hatran, Shannon Bragg, and Larkin Rieke for technical assistance, and Brenda Kreutzer for assistance with preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by U.S. Public Health Service Grant HD-03807.
Rights and permissions
About this article
Cite this article
Tomimatsu, T., Peňa, J. & Longo, L. Fetal Hypercapnia and Cerebral Tissue Oxygenation: Studies in Near-Term Sheep. Pediatr Res 60, 711–716 (2006). https://doi.org/10.1203/01.pdr.0000246308.37154.ce
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.pdr.0000246308.37154.ce