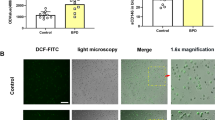
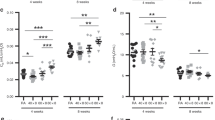
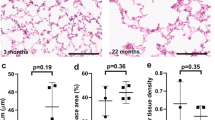
Abstract
Bronchopulmonary dysplasia (BPD) is a chronic lung disease that occurs in very premature infants and is characterized by impaired alveologenesis. This ultimate phase of lung development is mostly postnatal and allows growth of gas-exchange surface area to meet the needs of the organism. Alveologenesis is a highly integrated process that implies cooperative interactions between interstitial, epithelial, and vascular compartments of the lung. Understanding of its underlying mechanisms has considerably progressed recently with identification of structural, signaling, or remodeling molecules that are crucial in the process. Thus, the pivotal role of elastin deposition in lung walls has been demonstrated, and many key control-molecules have been identified, including various transcription factors, growth factors such as platelet-derived growth factor, fibroblast growth factors, and vascular endothelial growth factor, matrix-remodeling enzymes, and retinoids. BPD-associated changes in lung expression/content have been evidenced for most of these molecules, especially for signaling pathways, through both clinical investigations in premature infants and the use of animal models, including the premature baboon or lamb, neonatal exposure to hyperoxia in rodents, and maternal-fetal infection. These findings open therapeutic perspectives to correct imbalanced signaling. Unraveling the intimate molecular mechanisms of alveolar building appears as a prerequisite to define new strategies for the prevention and care of BPD.
Similar content being viewed by others
Main
BPD was initially defined as a disorder occurring in infants ventilated for neonatal respiratory distress; the described features included mucosal metaplasia of airways, emphysema, and widespread interstitial fibrosis (1). Over the years, probably as a consequence of both progress in therapeutic strategies and survival in greater proportion of highly premature infants, BPD has been characterized by a reduced frequency of airway injury, but an increase in alveolar growth disorders (2,3). This led to a “new” definition of BPD in which impairment of alveolar formation is the prominent feature, leading to long-term global reduction in alveolar number and gas-exchange surface area (4,5). BPD is now considered as resulting from the impact of injury, including oxygen toxicity, barotrauma/volutrauma, and infection, on a very immature lung, which leads in turn to arrest of normal maturation (6), with possible variable susceptibility due to some gene polymorphisms. Prenatal injury consecutive to glucocorticoid exposure or chorioamnionitis may also be involved (7). Unlike injury to the adult lung that is essentially growth arrested, BPD indeed occurs in a growing lung with uncompleted morphogenesis. The formation of definitive alveoli by secondary septation of primitive saccules is effectively an essentially postnatal event: if the alveolar phase of human lung development extends from about 36 wk gestation to 18 mo postnatally, the majority of alveologenesis (various synonym terms have been used to designate the process, including alveogenesis, alveologenesis, alveolization, and alveolarization, of which alveologenesis and alveolization are the most correct etymologically) occurs within 5–6 mo of term birth (8). Infants susceptible to develop BPD are therefore born in the early saccular phase, or even in the canalicular phase of lung development for the most premature of them (8). The pathophysiological mechanisms leading to BPD have been appraised at a variety of levels including cell proliferation (9), inflammation and fibrotic process (10), oxidative stress (11), infection (12), or microvascular development (13). The purpose of the present review is to focus on recent knowledge about the control mechanisms of alveolar septa formation and their impairments in BPD and its animal models. Much information has effectively been gained from in vivo models that reproduce abnormalities encountered in BPD. The closest model to the human condition is probably the prematurely delivered, ventilated baboon (14). In rodents that present totally postnatal alveologenesis (between d 4 and 20), neonatal exposure to hyperoxia that inhibits septal formation and affects alveologenesis (15, 16) has been widely used for more than 20 years as a model to study associated cell and molecular alterations. Experimental chorioamnionitis has also been developed (17), although used to a lesser extent.
Complexity in studying alveologenesis arises from the fact that the process is coordinated by multiple interactions through paracrine mechanisms between the fibroblastic, epithelial, and microvascular lung components, and with extracellular matrix (ECM). Defects in one of these components have repercussions on the whole alveolar development. The basis for intervention to prevent or reverse impaired alveologenesis depends on clarification of these complex interrelationships that are operative during normal lung development.
ELASTOGENESIS IS ESSENTIAL TO ALVEOLAR SEPTATION
To provide gas-exchange efficiently in the postnatal organism, the lung undergoes dramatic tissue growth and remodeling. The formation of new interalveolar walls known as alveolar septation is a necessary step to increase blood-gas interface and meet the respiratory requirements of the growing organism. Among the variety of factors that participate in the control of budding of secondary septa, elastin deposition in the thickness of primary septa appears to have a spatially instructive role inasmuch as the specific sites of elastic fiber formation correspond precisely to the location of future buds. New septa then extend that are composed of a double capillary layer, and elastin localizes at their tip (8). Later in development, microvascular maturation takes place with fusion of the double capillary layer into a single medial layer facing both alveolar lumens of the septum (8), and alveolar walls become thinner by apoptosis (18). Elastin is elaborated by cross-linking of a soluble precursor, tropoelastin, under the action of lysyl oxidase. Septal tropoelastin is produced by interstitial cells that express smooth-muscle actin, and are designated myofibroblasts.
The essential role of elastin for distal lung development was evidenced by different approaches. Although the lungs of mice devoid of elastin gene developed until the saccular stage, they displayed fewer, dilated distal air sacs with attenuated tissue septa, a condition reminiscent of emphysema (19). However, evaluating the consequences for secondary septation was not possible using this model, inasmuch as elastin null mice did not survive beyond the 4th postnatal day. The requirement for elastin deposition was indirectly evidenced by invalidation of platelet-derived growth factor A (PDGFA) gene. In this model, a profound deficiency in alveolar myofibroblasts and associated bundles of elastin fibers resulted in absence of secondary septa and definitive alveoli (20,21). Importantly, the loss of myofibroblast staining and elastin was limited to the lung parenchyma, whereas vascular and bronchial smooth muscle cells with accompanying elastin deposition were clearly observed, which emphasizes the specificity of septal elastogenesis blockade (20,21). It seems that this was due to a failure of myofibroblasts or their precursors to migrate from more proximal to the peripheral sites of the lung where alveolar elastin deposits should occur. These observations evidence a crucial role of PDGFA, which is produced by epithelial cells, as a chemoattractant for fibroblasts before the onset of septation (21). It has been suggested that this migration to sites of septal budding is not a random phenomenon, but conversely, that a morphogen gradient would provide instruction for the precise and specific localization of septa (22). Retinoic acid (see “Alveolar Formation Is Antagonistically Influenced by RA and GC”) and sonic hedgehog have been proposed as candidate molecules for establishing such a gradient that would in turn lead to precise regulation of PDGFA production to provide appropriate guidance to myofibroblasts (22). These interrelationships are depicted in Figure 1, which tentatively summarizes the various cell–cell and cell–matrix interactions involved in the control of alveolization process.
Elastin was found to be increased in ventilated infants who died of BPD (23). Elastin deposition and expression were also enhanced in a ventilated preterm lamb model (24). These findings, however, may relate to the fibrotic repair process prominent in “old” BPD. Some confusion may effectively arise from the fact that myofibroblasts are essential as a unique source of connective tissue material in the normal process of septa formation but are also involved in the fibrotic process that often occurs in the reparative phase of lung injury. Furthermore, increased elastin turnover (25) and paucity of elastic fibers in alveolar walls because of destruction consecutive to imbalance between protease and antiprotease activities (26) have also been reported in BPD. In rat pups exposed to hyperoxia during alveologenesis, disruption of elastin fibers was also found (27), but this went along with decreased tropoelastin expression (28). Whether changes in septal elastin deposition occur in early stages of BPD is unknown and calls for further investigation.
FGF signaling is also critical for alveologenesis. FGF receptors (FGFR) 1–4 are expressed in the developing lung with specific spatial and temporal profiles. Alveolar formation coincides with increased expression of FGFR3 and 4 (29). Mice devoid of both FGFR3 and 4 manifest a failure of secondary septation not observed in either single mutant (30). The underlying mechanisms have not yet been cleared up. FGF18, which is produced by fibroblasts (Fig. 1), may represent one of the implicated ligands acting in an autocrine manner, because, on the one hand, its expression is markedly increased concomitantly with alveolar septation, and, on the other hand, FGF18 enhanced myofibroblast growth and expression of tropoelastin and lysyl oxidase (31). Moreover, elastogenesis also involves the synthesis of microfibril proteins such as fibrillins and fibulins that act as scaffold for elastin assembly and are essential to the process (32,33). Their pulmonary expression is up-regulated during alveologenesis (34), and FGF18 stimulated expression of fibulins 1 and 5 in myofibroblasts (31). Nevertheless, elastin deposition in primary septa occurred in the FGFR3/4 null mice and even failed to cease with aging (30). Therefore, other FGF-driven mechanisms must occur that i) condition septal surge and ii) stop elastogenesis. FGF2 might constitute an attractive candidate for the latter process, based on its negative effect on tropoelastin production in neonatal lung fibroblasts in vitro (35). Because no lung abnormality was reported in FGF2 null mice, the involvement of other mediators is, however, likely.
Little is known about the status of peptide factors that control alveolar septation and/or elastogenesis in BPD and its models. Delayed expression of PDGFA (36) and reduced expression of FGFR4 (37) were observed in the lung of rat neonates exposed to hyperoxia, but these molecules have thus far not been explored in infants with BPD. Although FGF2 was found to be elevated in tracheal aspirates of preterm neonates who died or developed BPD and to correlate with apoptosis (38), it is not known whether this participated to the outcome of BPD. By contrast, a decrease of FGF2 followed by an increase under recovery in air were observed in neonatal rats exposed to 95% oxygen, and, interestingly, intraperitoneal injection of soluble inactive FGFR1, which is a receptor of FGF2, arrested compensatory lung growth and secondary septation in recovering animals (39). These findings suggest a role of FGF2 in repair process.
OTHER ECM COMPONENTS, ECM REMODELING, AND INTERACTIONS WITH MEDIATORS
Alveolar septation also implies the deposition of other ECM components, including collagens and proteoglycans (Fig. 1), as well as activity of enzymes that elaborate the carbohydrate components of the latter. Their gene expression is up-regulated in the lung postnatally (34). Moreover, the relative lung contents of collagen and fibronectin markedly increase coincidentally with alveolar septation (40,41). Collagen architecture was markedly distorted in infants with BPD, with thickened, tortuous, and disorganized fibers (40). Fibronectin mRNA and protein were increased and decreased in early acute and chronic phases of BPD, respectively (42), but the specimens in this study were characteristic of “old” BPD.
ECM remodeling is an important parameter of harmonious pulmonary development. MMP2, also designated gelatinase A, appears to play particularly important role in the postnatal lung. In the rat, MMP2 activity was maximal in the first 11 d, and the major part of the enzyme was present in the active form (43). In addition to defects in branching morphogenesis, MMP2 null mice exhibited abnormal saccular development, and similar features were found to be associated with low expression of MT1-MMP, an activator of MMP2, in mice lacking EGF receptor (44). Moreover, the dynamic induction of MMP2 seen in neonatal lungs during the first days of life was significantly impacted by hyperoxia (45). Consistent with the assumption of a major role of MMP2 in septation process, lowered MMP2 activity appears as a characteristic feature of infants who develop BPD, during the initial, acute phase of the disease (46–48). Elevated levels of the other gelatinase, MMP9, and high MMP9/TIMP1 ratio rather appear to be associated with the later, regenerative and chronic phases of the disease, and with fibrosis (48,49). A similar pattern was found in the extremely premature ventilated baboon (50).
Lastly, ECM has not only structural properties, but functions as a dynamic modulator through the selective sequestration and subsequent release of growth factors and cytokines. A striking example of the importance of this function for alveologenesis is given by disturbance of alveolar septation despite normal lung cell differentiation, including myofibroblasts, in mice deficient in fibrillin-1 (51). This appears likely to result from enhanced proportion of active TGF-β through greater local activation (51). Imbalanced production of this cytokine effectively appears as a major mechanism in the pathogenesis of BPD. Increased levels of TGF-β have been detected in airway secretions of preterm infants with BPD (52). In animal models developed to enhance endogenous production of TGF-β (53), or to induce TGF-β overexpression (54,55), pulmonary morphologic changes consistent with those seen in human BPD have been observed, including enlarged alveolar sacs, poor secondary septation, thick and hypercellular septa, and decreased platelet endothelial cell adhesion molecule (PECAM) expression indicative of abnormal capillary development. The adverse effects of TGF-β on septation appear paradoxical inasmuch as TGF-β is known to up-regulate elastin production and gene expression in alveolar fibroblasts (56,57). Possibly, myofibroblast proliferative effects and defects in angiogenesis may be determinant (see “Vascular Growth Is Required for Normal Alveolar Development”).
DEFECTS IN ALVEOLAR DEVELOPMENT CAN RESULT FROM ALTERED RESPIRATORY EPITHELIAL CELL GROWTH AND DIFFERENTIATION
Alveologenesis is characterized by an extensive proliferation of alveolar type II (ATII) cells. Sufficient ATII cell number is important because they serve as stem cells for alveolar type I (ATI) cells that line most of the alveolar surface and form air-blood barriers, and because they ensure adequate surfactant production around birth. Conditional deletion of the winged helix transcription factor Foxa2 (or HNF3β) has evidenced its requirement for ATII cell differentiation (58). Moreover, when Foxa2 was deleted in late gestation, extensive airspace enlargement and altered septation were displayed (59). Full ATII cell differentiation therefore appears to be required for alveolar septation, illustrating the concept that epithelial signals are essential to the interstitial events of alveologenesis. The zinc-finger transcription factor GATA6 is also necessary to fetal lung maturation, including differentiation of both ATI and ATII cells (60), but, paradoxically, maintenance of elevated transcription of GATA6 in mice during the postnatal period impaired alveolar septation (61). Control of Foxa2 and GATA6 expression levels appears essential for regulating the expression of genes involved in lung development both through up and down transcriptional regulations. Last, it is worth mentioning that in mice null for T1α, an ATI cell surface marker, abnormal distal lung cell proliferation, and narrower and irregular air spaces were observed at birth (62). However, the underlying mechanism is unknown.
Among growth factors acting on alveolar epithelial cells, FGF7 (or keratinocyte growth factor), whoch is released by lung fibroblasts (Fig. 1) has early been recognized as a potent proliferation stimulus for adult ATII (63). This mediator has also a potent stimulatory effect on proliferation and maturation of developing ATII cells (64). In tracheal aspirates from premature neonates within 5 d after birth, FGF7 was found to be significantly higher in survivors without BPD than in those with BPD; a concentration higher than 110 pg/mL had a positive predictive value of 95% for absence of BPD (65). FGF7 has also been shown to prevent lung epithelial injury induced by different forms of aggressions, including oxidative stress (66–68), mechanical ventilation (69), and infection (70), which emphasizes its key regulatory role for the alveolar epithelial compartment. Consistently, transgenesis of a soluble FGF receptor that bound FGF7 rendered mice more susceptible to hyperoxia (71). However, FGF7 failed to protect against hyperoxic inhibition of postnatal alveolar formation and early pulmonary fibrosis in newborn rats (67). Because altered cyclin and Cdk expression consistent with G1 or G2 arrest has been reported in epithelial cells in the premature baboon model of BPD (72), FGF7 might reveal useful to enhance ATII proliferation. Interestingly, FGF7 induced new alveolar formation after pneumonectomy in adult lungs (73). Taken together, these investigations not only suggest that FGF7 determination may help evaluating the risk for BPD in preemies but that supplying exogenous FGF7 may protect the alveolar epithelium from BPD-associated injuries.
Another aspect of epithelial-interstitial cell interactions in alveologenesis and BPD is the involvement of IGFs. Leprechaunism, a disease caused by a defect of IGF-I receptor (IGF-IR), is associated with reduced lung surface area and larger, less numerous alveoli (74). Consistently, it has recently been reported that alveolar development correlates with IGF-I level: comparison between normal and dexamethasone- or retinoic-acid-treated neonatal rats indicated that the stronger the IGF-I and -II expression, the better the alveolar development (75). Moreover, IGF-I produced by epithelial cells (Fig. 1) stimulated in vitro migration and proliferation of lung fibroblasts (76). However, hyperoxia enhanced IGF-I and -II expression in neonatal rat lung in vivo (77) and in explant cultures (78). Simultaneously, IGF-IR were increased in fibroblasts (78). IGF-I in epithelium and IGF-IR in myofibroblasts were also intensely increased in the lung of patients with BPD (79). The significance of these findings is therefore not fully clear. Presumably, IGF-I is a positive enhancer of alveolar development, and its increase in BPD is associated with repair process rather than with pathogenesis of the disease.
VASCULAR GROWTH IS REQUIRED FOR NORMAL ALVEOLAR DEVELOPMENT
VEGF signaling plays a major role for microvascular lung development (13). VEGF is released principally by respiratory epithelial cells and enhances migration, proliferation, and differentiation of adjacent endothelial cells via paracrine signaling to receptors Flt-1 and Flk-1 (Fig. 1). The requirement of normal angiogenesis for alveologenesis has been demonstrated by the use in the developing rat of angiogenesis inhibitors, including VEGF receptor inhibitor of neutralizing antibody (80,81,81a). These inhibitors not only impaired pulmonary vascular growth, but also reduced septation and final alveolar number. Nitric oxide (NO) is a downstream regulator of VEGF, and, interestingly, NO synthase was considerably reduced after treatment with VEGF receptor inhibitor, whereas inhaled NO corrected alveolar disorders in this model (82). The heparan-sulfate-binding isoform VEGF188, which strongly increases its expression shortly before birth (83,84), appears especially important inasmuch as mice only expressing the freely diffusible VEGF120 isoform presented at birth reduced peripheral airspaces and microvasculature, with fewer air-blood barriers (85) The essential role of VEGF signaling for the maintenance of alveolar structure was also evidenced by occurrence of emphysema in adult rats treated with VEGF receptor inhibitor (86), and in adult mice with lung-targeted VEGF inactivation (87). However, VEGF overexpression in neonatal mouse lung increased mortality and caused pulmonary hemorrhage, hemosiderosis, alveolar remodeling, and inflammation (88), which indicates that, although being necessary for postnatal lung development, VEGF expression must be strictly controlled.
Impairments of growth, structure, and function of the developing pulmonary vessels in BPD and models have been extensively reviewed recently (13). Recent quantitative analysis has showed that infants with BPD present fewer air-blood barriers, less capillary loading, and more distant capillaries from the air surface than controls (89). Over time, however, primary septal walls adapt by thinning and increasing the number of air-blood barriers, thereby taking on the function of secondary septa (89). It is clear that vascular disorders result at least partly from altered signaling of angiogenic factors, their receptors, and NO synthase, as evidenced in human infants (90,91), in prematurely delivered baboons (92), in lambs exposed to intra-amniotic endotoxin (93) and in hyperoxia-exposed rat neonates (37). It is worth emphasizing that VEGF signaling was primarily affected, whereas angiopoietin–1, another angiogenic growth factor, and its receptor Tie2 were unchanged in the baboon model (92). Decreased Tie2 was observed in human infants with BPD, however (90). Similarly, hyperoxic lung injury in newborn rats reduced expression of VEGF, VEGF receptors, and HIF2α, a transcription factor involved in the control of VEGF expression (94). It also reduced the VEGF188 isoform in newborn rabbits (83). Last, providing inhaled NO to premature infants slightly decreased the incidence of BPD and death (95), presumably through VEGF production by epithelial cells and subsequent protection of lung vascular development.
On the other hand, EMAP II, an anti-angiogenic protein distributed to regions of epithelial-mesenchymal interactions (Fig. 1), may play a functional role during alveolar development as a putative negative regulator of vessel formation (96). Importantly, EMAP II is maintained at low expression level throughout postnatal life and in the adult, with the exception of a surge that correlates with microvascular maturation (96), which suggests that vascular growth must be down-regulated when fusion of the double capillary network into a single one occurs. Recently, it was reported that EMAP II abundance is elevated in the lung tissue of infants with BPD as well as in the premature baboon model (97), suggesting that this protein may contribute to the interruption of vascular development seen in BPD.
ALVEOLAR FORMATION IS ANTAGONISTICALLY INFLUENCED BY RA AND GC
Before septation, the lung contains a relatively large supply of vitamin A under the form of retinyl esters. These precursors are stored in lipid interstitial cells, a subset of fibroblasts concentrated at sites of alveolus formation, which convert them into RA (Fig. 1) (98). The RA-synthesizing enzymes aldehyde dehydrogenase 1 (Aldh-1) and retinaldehyde dehydrogenase 2 (Raldh-2) are up-regulated during the period of maximal alveolar-wall cell proliferation (99). RA enhances tropoelastin gene expression (100), and using inhibitors of Aldh-1, Raldh-2, and retinyl ester hydrolases, it has been demonstrated that endogenous retinoids increase the steady state level of tropoelastin transcripts in rat lung fibroblasts and fetal lung explants (101). Retinoid involvement in the control of septation was evidenced by several experimental approaches. In neonatal rat pups, RA enhanced ongoing alveologenesis by increasing the total number of alveoli (102), whereas vitamin A deficiency led to delayed alveolar development (103). Furthermore, simultaneous deletion of two RA receptors, RARγ and RXRα (104), or overexpression of dominant negative RARα (105) reduced alveolar number, whereas RARβ knockout mice exhibited higher alveolar number (106). These findings support the concept that endocrine RA and its receptors RARs/RXRs play a complex and critical role in alveolization during the neonatal period of the lung, including both stimulatory and inhibitory influences. In addition to elastin, RA or retinol have been shown to stimulate the expression of PDGFA/PDGFRα (107,108) and FGF18 (31). Finally, RA abrogated key features of emphysema in an elastase-generated model of the disease (109) that highlights the regenerative properties of RA and suggests that developmental and repair processes share common regulatory mechanisms.
Blood retinol concentration has early been recognized to be lower in prematurely born than in full-term infants (110), and in those who develop BPD than in those who do not (111,112). In trials of vitamin A supplementation, reduced need for supplemental oxygen and mechanical ventilation was observed (113), but whether the incidence of BPD was reduced remains controversial with either no change (114) or slight decrease (115). As regards direct administration of RA, only experimental approaches in the hyperoxic rat model have been performed. RA treatment of rats exposed to hyperoxia from postnatal d 3 increased collagen in airspace walls and mean alveolar area, but neither improved septal formation and microvessel count, nor decreased airspace size on d 14 or 18 (116,117). By contrast, on d 45, lungs were no longer different from those in controls, indicating complete recovery, whereas deficient alveologenesis remained obvious in hyperoxia-exposed rats that were not RA treated (118).
Although GC hormones are widely used in preemies to accelerate maturation and surfactant synthesis and to prevent inflammatory process, they appear to exert deleterious effects on alveologenesis. The postnatal formation of alveoli is largely prevented by GC treatment, which accelerates alveolar wall thinning, fusion of the two capillary layers, and inhibits outgrowth of new septa leading to early termination of the septation process as shown in monkeys (119), rats (120,121) or lambs (122,123). Importantly, RA treatment antagonized GC effects and partially rescued failed septation induced by a GC hormone in mice and rats (102,124). The underlying mechanisms of these antagonistic effects are not completely understood. GC stimulate developing-lung elastogenesis (125), which may appear paradoxical in view of their effects on septation. However, this stimulation is unlikely to relate to alveolar-wall elastin inasmuch as dexamethasone was reported to prevent alveolar elastin deposition (126). GC-induced inhibition of septation in mice was shown to be associated with a block in angiogenesis due to down-regulation of VEGFR2, and this down-regulation was prevented by RA treatment (127). Recent observations indicated that RA enhanced and dexamethasone decreased IGF-I (75) and midkine (128) in rat lung. In addition, midkine expression was suppressed in neonatal mice exposed to hyperoxia (129). Taken together with the above-reported probable importance of IGF-I in alveologenesis, and with the influence of midkine on pulmonary vascular gene expression (130), these findings indicate possible links between GC and RA accounting for their antagonism.
Balance between RA and GC occurs in the course of normal lung development, and disturbances in this equilibrium may lead to abnormal alveolization (7). However, survival was enhanced in oxygen-exposed newborn rats by simultaneous treatment with RA and dexamethasone (116), which suggests possible complementary effects also. RA may compensate the accelerating effects of GC on septal maturation while keeping the benefit of GC treatment for lung epithelial maturation and for lowering inflammation. Consistent with this assumption, it was observed that blood vitamin A level was higher in premature ventilated infants whom respiratory function positively responded to GC as compared with those who displayed no respiratory improvement (131).
CONCLUSION AND PERSPECTIVES
The spotlight focusing on impaired septation as a prominent feature of BPD prompts the neonatologist to question about disorders in the underlying molecular mechanisms. Although numerous elements in the process of alveolization have probably still to be determined, key control factors have already been identified during the last 10 years that indeed exhibit expression disturbances in BPD and/or models. Table 1 recapitulates the principal factors involved. Genes that regulate alveolar morphogenesis must be differentially expressed during periods of active and inactive alveolar formation. Global analyses of lung transcriptome or proteome that are increasingly developing in this field should therefore offer new prospects in the identification of candidate genes and help reaching a more comprehensive view of the process. Restoring balanced levels through supply of insufficient factors and inhibition of excessive factors appears as a promising clinical approach for prevention or treatment of the disease. However, it should be stressed that alveolization, like other developmental processes, undoubtedly depends on complex interactions that are normally tightly controlled and balanced in time and space. The antagonistic effects of glucocorticoids and retinoids and the possible harmful consequences of incautious use of glucocorticoids are especially illustrative in this respect. Future attempts to intervene in these processes must take into account not only the potential benefit of supplementing the immature organism with exogenous factors but also the serious potential for unanticipated adverse effects on both the lung and other organ systems.
Abbreviations
- BPD:
-
bronchopulmonary dysplasia
- ECM:
-
extracellular matrix
- EMAP II:
-
endothelial-monocyte activating polypeptide II
- FGF:
-
fibroblast growth factor
- GC:
-
glucocorticoids
- MMP:
-
matrix metalloproteinase
- PDGF:
-
platelet-derived growth factor
- RA:
-
retinoic acid
- TGF:
-
transforming growth factor
- VEGF:
-
vascular endothelial growth factor
- TIMP:
-
tissue inhibitor of metalloproteinases
References
Northway WH Jr, Rosan RC, Porter DY 1967 Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med 276: 357–368
Rojas MA, Gonzalez A, Bancalari E, Claure N, Poole C, Silva-Neto G 1995 Changing trends in the epidemiology and pathogenesis of neonatal chronic lung disease. J Pediatr 126: 605–610
Chambers HM, van Velzen D 1989 Ventilator-related pathology in the extremely immature lung. Pathology 21: 79–83
Husain AN, Siddiqui NH, Stocker JT 1998 Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717
Jobe A 1999 The new BPD: an arrest of lung development. Pediatr Res 46: 641–643
Jobe AH, Bancalari E 2001 Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163: 1723–1729
Jobe AH 2003 Antenatal factors and the development of bronchopulmonary dysplasia. Semin Neonatol 8: 9–17
Burri PH 1997 Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald JA (ed) Lung Growth and Development. Marcel Dekker, New York, pp 1–35
Jankov RP, Tanswell AK 2004 Growth factors, postnatal lung growth and bronchopulmonary dysplasia. Pediatr Respir Rev 5: S265–S275
Speer CP 2003 Inflammation and bronchopulmonary dysplasia. Semin Neonatol 8: 29–38
Saugstad OD 2003 Bronchopulmonary dysplasia-oxidative stress and antioxidants. Semin Neonatol 8: 39–49
Li YH, Tullus K 2002 Microbial infection and inflammation in the development of chronic lung disease of prematurity. Microbes Infect 4: 723–732
Stenmark KR, Abman SH 2005 Lung vascular development: implications for the pathogenesis of bronchopulmonary dysplasia. Annu Rev Physiol 67: 623–661
Coalson JJ, Winter VT, Siler-Khodr T, Yoder BA 1999 Neonatal chronic lung disease in extremely immature baboons. Am J Respir Crit Care Med 160: 1333–1346
Randell SH, Mercer RR, Young SL 1989 Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat 186: 55–68
Warner BB, Stuart LA, Papes RA, Wispe JR 1998 Functional and pathological effects of prolonged hyperoxia in neonatal mice. Am J Physiol 275: L110–L117
Kramer BW, Kramer S, Ikegami M, Jobe AH 2002 Injury, inflammation, and remodeling in fetal sheep lung after intra-amniotic endotoxin. Am J Physiol Lung Cell Mol Physiol 283: L452–L459
Bruce MC, Honaker CE, Cross RJ 1999 Lung fibroblasts undergo apoptosis following alveolarization. Am J Respir Cell Mol Biol 20: 228–236
Wendel DP, Taylor DG, Albertine KH, Keating MT, Li DY 2000 Impaired distal airway development in mice lacking elastin. Am J Respir Cell Mol Biol 23: 320–326
Bostrom H, Willetts K, Pekny M, Leveen P, Lindahl P, Hedstrand H, Pekna M, Hellstrom M, Gebre-Medhin S, Schalling M, Nilsson M, Kurland S, Tornell J, Heath JK, Betsholtz C 1996 PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell 85: 863–873
Lindahl P, Karlsson L, Hellstrom M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C 1997 Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development 124: 3943–3953
Prodhan P, Kinane TB 2002 Developmental paradigms in terminal lung development. Bioessays 24: 1052–1059
Thibeault DW, Mabry SM, Ekekezie II, Truog WE 2000 Lung elastic tissue maturation and perturbations during the evolution of chronic lung disease. Pediatrics 106: 1452–1459
Pierce RA, Albertine KH, Starcher BC, Bohnsack JF, Carlton DP, Bland RD 1997 Chronic lung injury in preterm lambs: disordered pulmonary elastin deposition. Am J Physiol 272: L452–L460
Bruce MC, Wedig KE, Jentoft N, Martin RJ, Cheng PW, Boat TF, Fanaroff AA 1985 Altered urinary excretion of elastin cross-links in premature infants who develop bronchopulmonary dysplasia. Am Rev Respir Dis 131: 568–572
Bruce MC, Schuyler M, Martin RJ, Starcher BC, Tomashefski JF Jr, Wedig KE 1992 Risk factors for the degradation of lung elastic fibers in the ventilated neonate. Implications for impaired lung development in bronchopulmonary dysplasia. Am Rev Respir Dis 146: 204–212
Bruce MC, Pawlowski R, Tomashefski JF Jr 1989 Changes in lung elastic fiber structure and concentration associated with hyperoxic exposure in the developing rat lung. Am Rev Respir Dis 140: 1067–1074
Bruce MC, Bruce EN, Janiga K, Chetty A 1993 Hyperoxic exposure of developing rat lung decreases tropoelastin mRNA levels that rebound postexposure. Am J Physiol 265: L293–L300
Powell PP, Wang CC, Horinouchi H, Shepherd K, Jacobson M, Lipson M, Jones R 1998 Differential expression of fibroblast growth factor receptors 1 to 4 and ligand genes in late fetal and early postnatal rat lung. Am J Respir Cell Mol Biol 19: 563–572
Weinstein M, Xu X, Ohyama K, Deng CX 1998 FGFR-3 and FGFR-4 function cooperatively to direct alveogenesis in the murine lung. Development 125: 3615–3623
Chailley-Heu B, Boucherat O, Barlier-Mur AM, Bourbon JR 2005 FGF-18 is up-regulated in the postnatal rat lung and enhances elastogenesis in myofibroblasts. Am J Physiol Lung Cell Mol Physiol 288: L43–L51
Trask TM, Trask BC, Ritty TM, Abrams WR, Rosenbloom J, Mecham RP 2000 Interaction of tropoelastin with the amino-terminal domains of fibrillin-1 and fibrillin-2 suggests a role for the fibrillins in elastic fiber assembly. J Biol Chem 275: 24400–24406
Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, Tashiro K, Ross J Jr, Honjo T, Chien KR 2002 Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature 415: 171–175
Mariani TJ, Reed JJ, Shapiro SD 2002 Expression profiling of the developing mouse lung: insights into the establishment of the extracellular matrix. Am J Respir Cell Mol Biol 26: 541–548
Brettell LM, McGowan SE 1994 Basic fibroblast growth factor decreases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol 10: 306–315
Buch S, Han RN, Cabacungan J, Wang J, Yuan S, Belcastro R, Deimling J, Jankov R, Luo X, Lye SJ, Post M, Tanswell AK 2000 Changes in expression of platelet-derived growth factor and its receptors in the lungs of newborn rats exposed to air or 60% O2 . Pediatr Res 48: 423–433
Wagenaar GT, ter Horst SA, van Gastelen MA, Leijser LM, Mauad T, van der Velden PA, de Heer E, Hiemstra PS, Poorthuis BJ, Walther FJ 2004 Gene expression profile and histopathology of experimental bronchopulmonary dysplasia induced by prolonged oxidative stress. Free Radic Biol Med 36: 782–801
Ambalavanan N, Novak ZE 2003 Peptide growth factors in tracheal aspirates of mechanically ventilated preterm neonates. Pediatr Res 53: 240–244
Jankov RP, Luo X, Campbell A, Belcastro R, Cabacungan J, Johnstone L, Frndova H, Lye SJ, Tanswell AK 2003 Fibroblast growth factor receptor-1 and neonatal compensatory lung growth after exposure to 95% oxygen. Am J Respir Crit Care Med 167: 1554–1561
Thibeault DW, Mabry SM, Ekekezie II, Zhang X, Truog WE 2003 Collagen scaffolding during development and its deformation with chronic lung disease. Pediatrics 111: 766–776
Plumb DJ, Dubaybo BA, Thet LA 1987 Changes in lung tissue fibronectin content and synthesis during postnatal lung growth. Pediatr Pulmonol 3: 413–419
Sinkin RA, Roberts M, LoMonaco MB, Sanders RJ, Metlay LA 1998 Fibronectin expression in bronchopulmonary dysplasia. Pediatr Dev Pathol 1: 494–502
Arden MG, Adamson IY 1992 Collagen degradation during postnatal lung growth in rats. Pediatr Pulmonol 14: 95–101
Kheradmand F, Rishi K, Werb Z 2002 Signaling through the EGF receptor controls lung morphogenesis in part by regulating MT1-MMP-mediated activation of gelatinase A/MMP2. J Cell Sci 115: 839–848
Buckley S, Warburton D 2002 Dynamics of metalloproteinase-2 and -9, TGF-beta, and uPA activities during normoxic vs. hyperoxic alveolarization. Am J Physiol Lung Cell Mol Physiol 283: L747–L754
Danan C, Jarreau PH, Franco ML, Dassieu G, Grillon C, Abd Alsamad I, Lafuma C, Harf A, Delacourt C 2002 Gelatinase activities in the airways of premature infants and development of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 283: L1086–L1093
Schulz CG, Sawicki G, Lemke RP, Roeten BM, Schulz R, Cheung PY 2004 MMP-2 and MMP-9 and their tissue inhibitors in the plasma of preterm and term neonates. Pediatr Res 55: 794–801
Ekekezie II, Thibeault DW, Simon SD, Norberg M, Merrill JD, Ballard RA, Ballard PL, Truog WE 2004 Low levels of tissue inhibitors of metalloproteinases with a high matrix metalloproteinase-9/tissue inhibitor of metalloproteinase-1 ratio are present in tracheal aspirate fluids of infants who develop chronic lung disease. Pediatrics 113: 1709–1714
Dik WA, De Krijger RR, Bonekamp L, Naber BA, Zimmermann LJ, Versnel MA 2001 Localization and potential role of matrix metalloproteinase-1 and tissue inhibitors of metalloproteinase-1 and -2 in different phases of bronchopulmonary dysplasia. Pediatr Res 50: 761–766
Tambunting F, Beharry KD, Hartleroad J, Waltzman J, Stavitsky Y, Modanlou HD 2005 Increased lung matrix metalloproteinase-9 levels in extremely premature baboons with bronchopulmonary dysplasia. Pediatr Pulmonol 39: 5–14
Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC 2003 Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411
Kotecha S, Wangoo A, Silverman M, Shaw RJ 1996 Increase in the concentration of transforming growth factor beta-1 in bronchoalveolar lavage fluid before development of chronic lung disease of prematurity. J Pediatr 128: 464–469
Vicencio AG, Eickelberg O, Stankewich MC, Kashgarian M, Haddad GG 2002 Regulation of TGF-beta ligand and receptor expression in neonatal rat lungs exposed to chronic hypoxia. J Appl Physiol 93: 1123–1130
Gauldie J, Galt T, Bonniaud P, Robbins C, Kelly M, Warburton D 2003 Transfer of the active form of transforming growth factor-beta 1 gene to newborn rat lung induces changes consistent with bronchopulmonary dysplasia. Am J pathol 163: 2575–2584
Vicencio AG, Lee CG, Cho SJ, Eickelberg O, Chuu Y, Haddad GG, Elias JA 2004 Conditional overexpression of bioactive transforming growth factor-beta1 in neonatal mouse lung: a new model for bronchopulmonary dysplasia?. Am J Respir Cell Mol Biol 31: 650–656
McGowan SE, McNamer R 1990 Transforming growth factor-beta increases elastin production by neonatal rat lung fibroblasts. Am J Respir Cell Mol Biol 3: 369–376
McGowan SE, Jackson SK, Olson PJ, Parekh T, Gold LI 1997 Exogenous and endogenous transforming growth factors-beta influence elastin gene expression in cultured lung fibroblasts. Am J Respir Cell Mol Biol 17: 25–35
Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, Whitsett JA 2004 Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A 101: 14449–14454
Wan H, Kaestner KH, Ang SL, Ikegami M, Finkelman FD, Stahlman MT, Fulkerson PC, Rothenberg ME, Whitsett JA 2004 Foxa2 regulates alveolarization and goblet cell hyperplasia. Development 131: 953–964
Liu C, Morrisey EE, Whitsett JA 2002 GATA-6 is required for maturation of the lung in late gestation. Am J Physiol Lung Cell Mol Physiol 283: L468–L475
Liu C, Ikegami M, Stahlman MT, Dey CR, Whitsett JA 2003 Inhibition of alveolarization and altered pulmonary mechanics in mice expressing GATA-6. Am J Physiol Lung Cell Mol Physiol 285: L1246–L1254
Ramirez MI, Millien G, Hinds A, Cao Y, Seldin DC, Williams MC 2003 T1alpha, a lung type I cell differentiation gene, is required for normal lung cell proliferation and alveolus formation at birth. Dev Biol 256: 61–72
Ulich TR, Yi ES, Longmuir K, Yin S, Biltz R, Morris CF, Housley RM, Pierce GF 1994 Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest 93: 1298–1306
Chelly N, Mouhieddine-Gueddiche OB, Barlier-Mur AM, Chailley-Heu B, Bourbon JR 1999 Keratinocyte growth factor enhances maturation of fetal rat lung type II cells. Am J Respir Cell Mol Biol 20: 423–432
Danan C, Franco ML, Jarreau PH, Dassieu G, Chailley-Heu B, Bourbon J, Delacourt C 2002 High concentrations of keratinocyte growth factor in airways of premature infants predicted absence of bronchopulmonary dysplasia. Am J Respir Crit Care Med 165: 1384–1387
Barazzone C, Donati YR, Rochat AF, Vesin C, Kan CD, Pache JC, Piguet PF 1999 Keratinocyte growth factor protects alveolar epithelium and endothelium from oxygen-induced injury in mice. Am J Pathol 154: 1479–1487
Frank L 2003 Protective effect of keratinocyte growth factor against lung abnormalities associated with hyperoxia in prematurely born rats. Biol Neonate 83: 263–272
Ray P, Devaux Y, Stolz DB, Yarlagadda M, Watkins SC, Lu Y, Chen L, Yang XF, Ray A 2003 Inducible expression of keratinocyte growth factor (KGF) in mice inhibits lung epithelial cell death induced by hyperoxia. Proc Natl Acad Sci U S A 100: 6098–6103
Welsh DA, Summer WR, Dobard EP, Nelson S, Mason CM 2000 Keratinocyte growth factor prevents ventilator-induced lung injury in an ex vivo rat model. Am J Respir Crit Care Med 162: 1081–1086
Viget NB, Guery BP, Ader F, Neviere R, Alfandari S, Creuzy C, Roussel-Delvallez M, Foucher C, Mason CM, Beaucaire G, Pittet JF 2000 Keratinocyte growth factor protects against Pseudomonas aeruginosa-induced lung injury. Am J Physiol Lung Cell Mol Physiol 279: L1199–L1209
Hokuto I, Perl AK, Whitsett JA 2004 FGF signaling is required for pulmonary homeostasis following hyperoxia. Am J Physiol Lung Cell Mol Physiol 286: L580–L587
Das KC, Ravi D 2004 Altered expression of cyclins and cdks in premature infant baboon model of bronchopulmonary dysplasia. Antioxid Redox Signal 6: 117–127
Kaza AK, Kron IL, Leuwerke SM, Tribble CG, Laubach VE 2002 Keratinocyte growth factor enhances post-pneumonectomy lung growth by alveolar proliferation. Circulation 106: I120–I124
Thurlbeck WM, Cooney TP 1988 Dysmorphic lungs in a case of leprechaunism: case report and review of literature. Pediatr Pulmonol 5: 100–106
Liu H, Chang L, Rong Z, Zhu H, Zhang Q, Chen H, Li W 2004 Association of insulin-like growth factors with lung development in neonatal rats. J Huazhong Univ Sci Technolog Med Sci 24: 162–165
Chetty A, Faber S, Nielsen HC 1999 Epithelial-mesenchymal interaction and insulin-like growth factors in hyperoxic lung injury. Exp Lung Res 25: 701–718
Veness-Meehan KA, Moats-Staats BM, Price WA, Stiles AD 1997 Re-emergence of a fetal pattern of insulin-like growth factor expression during hyperoxic rat lung injury. Am J Respir Cell Mol Biol 16: 538–548
Chetty A, Nielsen HC 2002 Regulation of cell proliferation by insulin-like growth factor 1 in hyperoxia-exposed neonatal rat lung. Mol Genet Metab 75: 265–275
Chetty A, Andersson S, Lassus P, Nielsen HC 2004 Insulin-like growth factor-1 (IGF-1) and IGF-1 receptor (IGF-1R) expression in human lung in RDS and BPD. Pediatr Pulmonol 37: 128–136
Jakkula M, Le Cras TD, Gebb S, Hirth KP, Tuder RM, Voelkel NF, Abman SH 2000 Inhibition of angiogenesis decreases alveolarization in the developing rat lung. Am J Physiol Lung Cell Mol Physiol 279: L600–L607
Le Cras TD, Markham NE, Tuder RM, Voelkel NF, Abman SH 2002 Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. Am J Physiol Lung Cell Mol Physiol 283: L555–L562
McGrath-Morrow SA, Cho C, Cho C, Zhen L, Hicklin DJ, Tuder RM 2005 VEGF receptor 2 blockade disrupts postnatal lung development. Am J Respir Cell Mol Biol Feb 18 [Epub ahead of print]
Tang JR, Markham NE, Lin YJ, McMurtry IF, Maxey A, Kinsella JP, Abman SH 2004 Inhaled nitric oxide attenuates pulmonary hypertension and improves lung growth in infant rats after neonatal treatment with a VEGF receptor inhibitor. Am J Physiol Lung Cell Mol Physiol 287: L344–L351
Watkins RH, D'Angio CT, Ryan RM, Patel A, Maniscalco WM 1999 Differential expression of VEGF mRNA splice variants in newborn and adult hyperoxic lung injury. Am J Physiol 276: L858–L867
Ng YS, Rohan R, Sunday ME, Demello DE, D'Amore PA 2001 Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn 220: 112–121
Galambos C, Ng YS, Ali A, Noguchi A, Lovejoy S, D‘Amore PA, DeMello DE 2002 Defective pulmonary development in the absence of heparin-binding vascular endothelial growth factor isoforms. Am J Respir Cell Mol Biol 27: 194–203
Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF 2000 Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest 106: 1311–1319
Tang K, Rossiter HB, Wagner PD, Breen EC 2004 Lung-targeted VEGF inactivation leads to an emphysema phenotype in mice. J Appl Physiol 97: 1559–1566
Le Cras TD, Spitzmiller RE, Albertine KH, Greenberg JM, Whitsett JA, Akeson AL 2004 VEGF causes pulmonary hemorrhage, hemosiderosis, and air space enlargement in neonatal mice. Am J Physiol Lung Cell Mol Physiol 287: L134–L142
Thibeault DW, Mabry SM, Norberg M, Truog WE, Ekekezie II 2004 Lung microvascular adaptation in infants with chronic lung disease. Biol Neonate 85: 273–282
Bhatt AJ, Pryhuber GS, Huyck H, Watkins RH, Metlay LA, Maniscalco WM 2001 Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt-1, and TIE-2 in human infants dying with bronchopulmonary dysplasia. Am J Respir Crit Care Med 164: 1971–1980
Lassus P, Turanlahti M, Heikkila P, Andersson LC, Nupponen I, Sarnesto A, Andersson S 2001 Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med 164: 1981–1987
Maniscalco WM, Watkins RH, Pryhuber GS, Bhatt A, Shea C, Huyck H 2002 Angiogenic factors and alveolar vasculature: development and alterations by injury in very premature baboons. Am J Physiol Lung Cell Mol Physiol 282: L811–L823
Kallapur SG, Bachurski CJ, Le Cras TD, Joshi SN, Ikegami M, Jobe AH 2004 Vascular changes after intra-amniotic endotoxin in preterm lamb lungs. Am J Physiol Lung Cell Mol Physiol 287: L1178–L1185
Hosford GE, Olson DM 2003 Effects of hyperoxia on VEGF, its receptors, and HIF-2alpha in the newborn rat lung. Am J Physiol Lung Cell Mol Physiol 285: L161–L168
Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P 2003 Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349: 2099–2107
Schwarz MA, Zhang F, Gebb S, Starnes V, Warburton D 2000 Endothelial monocyte activating polypeptide II inhibits lung neovascularization and airway epithelial morphogenesis. Mech Dev 95: 123–132
Quintos-Alagheband ML, White CW, Schwarz MA 2004 Potential role for antiangiogenic proteins in the evolution of bronchopulmonary dysplasia. Antioxid Redox Signal 6: 137–145
Dirami G, Massaro GD, Clerch LB, Ryan US, Reczek PR, Massaro D 2004 Lung retinol storing cells synthesize and secrete retinoic acid, an inducer of alveolus formation. Am J Physiol Lung Cell Mol Physiol 286: L249–L256
Hind M, Corcoran J, Maden M 2002 Alveolar proliferation, retinoid synthesizing enzymes, and endogenous retinoids in the postnatal mouse lung. Different roles for Aldh-1 and Raldh-2. Am J Respir Cell Mol Biol 26: 67–73
Liu B, Harvey CS, McGowan SE 1993 Retinoic acid increases elastin in neonatal rat lung fibroblast cultures. Am J Physiol Lung Cell Mol Physiol 265: L430–L437
McGowan SE, Doro MM, Jackson SK 1997 Endogenous retinoids increase perinatal elastin gene expression in rat lung fibroblasts and fetal explants. Am J Physiol 273: L410–L416
Massaro GD, Massaro D 1996 Postnatal treatment with retinoic acid increases the number of pulmonary alveoli in rats. Am J Physiol 270: L305–L310
Frey G, Egli E, Chailley-Heu B, Lelievre-Pegorier M, Burri PH, Bourbon J, Tschanz SA 2004 Effects of mild vitamin A deficiency on lung maturation in newborn rats: a morphometric and morphologic study. Biol Neonate 86: 259–268
McGowan S, Jackson SK, Jenkins-Moore M, Dai HH, Chambon P, Snyder JM 2000 Mice bearing deletions of retinoic acid receptors demonstrate reduced lung elastin and alveolar numbers. Am J Respir Cell Mol Biol 23: 162–167
Yang L, Naltner A, Yan C 2003 Overexpression of dominant negative retinoic acid receptor alpha causes alveolar abnormality in transgenic neonatal lungs. Endocrinology 144: 3004–3011
Massaro GD, Massaro D, Chan WY, Clerch LB, Ghyselinck N, Chambon P, Chandraratna RA 2000 Retinoic acid receptor-beta: an endogenous inhibitor of the perinatal formation of pulmonary alveoli. Physiol Genomics 4: 51–57
Liebeskind A, Srinivasan S, Kaetzel D, Bruce M 2000 Retinoic acid stimulates immature lung fibroblast growth via a PDGF-mediated autocrine mechanism. Am J Physiol Lung Cell Mol Physiol 279: L81–L90
Chen H, Chang L, Liu H, Rong Z, Zhu H, Zhang Q, Li W 2004 Effect of retinoic acid on platelet-derived growth factor and lung development in newborn rats. J Huazhong Univ Sci Technolog Med Sci 24: 226–228
Massaro GD, Massaro D 1997 Retinoic acid treatment abrogates elastase-induced pulmonary emphysema in rats. Nat Med 3: 675–677
Shenai JP, Chytil F, Jhaveri A, Stahlman MT 1981 Plasma vitamin A and retinol-binding protein in premature and term neonates. J Pediatr 99: 302–305
Hustead VA, Gutcher GR, Anderson SA, Zachman RD 1984 Relationship of vitamin A (retinol) status to lung disease in the preterm infant. J Pediatr 105: 610–615
Shenai JP, Chytil F, Stahlman MT 1985 Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatr Res 19: 185–188
Shenai JP, Kennedy KA, Chytil F, Stahlman MT 1987 Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr 111: 269–277
Pearson E, Bose C, Snidow T, Ransom L, Young T, Bose G, Stiles A 1992 Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr 121: 420–427
Tyson JE, Wright L, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CR, Korones SB, Fanaroff AA 1999 Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med 340: 1962–1968
Veness-Meehan KA, Bottone FG Jr, Stiles AD 2000 Effects of retinoic acid on airspace development and lung collagen in hyperoxia-exposed newborn rats. Pediatr Res 48: 434–444
Ozer EA, Kumral A, Ozer E, Duman N, Yilmaz O, Ozkal S, Ozkan H 2005 Effect of retinoic acid on oxygen-induced lung injury in the newborn rat. Pediatr Pulmonol 39: 35–40
Veness-Meehan KA, Pierce RA, Moats-Staats BM, Stiles AD 2002 Retinoic acid attenuates O2-induced inhibition of lung septation. Am J Physiol Lung Cell Mol Physiol 283: L971–L980
Bunton TE, Plopper CG 1984 Triamcinolone-induced structural alterations in the development of the lung of the fetal rhesus macaque. Am J Obstet Gynecol 148: 203–215
Massaro D, Massaro GD 1986 Dexamethasone accelerates postnatal alveolar wall thinning and alters wall composition. Am J Physiol Regul Integr Comp Physiol 251: R218–R224
Tschanz SA, Damke BM, Burri PH 1995 Influence of postnatally administered glucocorticoids on rat lung growth. Biol Neonate 68: 229–245
Willet KE, McMenamin P, Pinkerton KE, Ikegami M, Jobe AH, Gurrin L, Sly PD 1999 Lung morphometry and collagen and elastin content: changes during normal development and after prenatal hormone exposure in sheep. Pediatr Res 45: 615–625
Willet KE, Jobe AH, Ikegami M, Kovar J, Sly PD 2001 Lung morphometry after repetitive antenatal glucocorticoid treatment in preterm sheep. Am J Respir Crit Care Med 163: 1437–1443
Massaro GD, Massaro D 2000 Retinoic acid treatment partially rescues failed septation in rats and in mice. Am J Physiol Lung Cell Mol Physiol 278: L955–L960
Pierce RA, Mariencheck WI, Sandefur S, Crouch EC, Parks WC 1995 Glucocorticoids upregulate tropoelastin expression during late stages of fetal lung development. Am J Physiol 268: L491–L500
Blanco LN, Frank L 1993 The formation of alveoli in rat lung during the third and fourth postnatal weeks: effect of hyperoxia, dexamethasone, and deferoxamine. Pediatr Res 34: 334–340
Clerch LB, Baras AS, Massaro GD, Hoffman EP, Massaro D 2004 DNA microarray analysis of neonatal mouse lung connects regulation of KDR with dexamethasone-induced inhibition of alveolar formation. Am J Physiol Lung Cell Mol Physiol 286: L411–L419
Kaplan F, Comber J, Sladek R, Hudson TJ, Muglia LJ, Macrae T, Gagnon S, Asada M, Brewer JA, Sweezey NB 2003 The growth factor midkine is modulated by both glucocorticoid and retinoid in fetal lung development. Am J Respir Cell Mol Biol 28: 33–41
Matsuura O, Kadomatsu K, Takei Y, Uchimura K, Mimura S, Watanabe K, Muramatsu T 2002 Midkine expression is associated with postnatal development of the lungs. Cell Struct Funct 27: 109–115
Reynolds PR, Mucenski ML, Le Cras TD, Nichols WC, Whitsett JA 2004 Midkine is regulated by hypoxia and causes pulmonary vascular remodeling. J Biol Chem 279: 37124–37132
Shenai JP, Mellen BG, Chytil F 2000 Vitamin A status and postnatal dexamethasone treatment in bronchopulmonary dysplasia. Pediatrics 106: 547–553
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bourbon, J., Boucherat, O., Chailley-Heu, B. et al. Control Mechanisms of Lung Alveolar Development and Their Disorders in Bronchopulmonary Dysplasia. Pediatr Res 57, 38–46 (2005). https://doi.org/10.1203/01.PDR.0000159630.35883.BE
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000159630.35883.BE
This article is cited by
-
MicroRNA 219-5p inhibits alveolarization by reducing platelet derived growth factor receptor-alpha
Respiratory Research (2021)
-
α1,3-Fucosyltransferase-IX, an enzyme of pulmonary endogenous lung stem cell marker SSEA-1, alleviates experimental bronchopulmonary dysplasia
Pediatric Research (2021)
-
Hyperoxia-induced S1P1 signaling reduced angiogenesis by suppression of TIE-2 leading to experimental bronchopulmonary dysplasia
Cell Biochemistry and Biophysics (2021)
-
S-endoglin expression is induced in hyperoxia and contributes to altered pulmonary angiogenesis in bronchopulmonary dysplasia development
Scientific Reports (2020)
-
Bone marrow stem cells accelerate lung maturation and prevent the LPS-induced delay of morphological and functional fetal lung development in the presence of ErbB4
Cell and Tissue Research (2020)