Abstract
The safety of dexamethasone for neonates has been questioned, partly because of its multiple unspecific effects on the immune system. Specific effects of dexamethasone on co-stimulatory and immune suppressive functions of neonatal compared with adult macrophages (MΦ) are not known. We evaluated the effect of dexamethasone on the expression and regulation of MΦ B7 family receptors (B7-1, CD80; B7-2, CD86) and on their ability to co-stimulate T cells. Cord blood macrophages (CBMΦ) and MΦ from healthy adults (PBMΦ) were isolated, and cell surface markers were phenotyped by flow cytometry. In tissue culture, cells were exposed to dexamethasone, interferon-γ (IFN-γ), cAMP, or a T cell mitogen (αCD3) and examined for their capacity to activate or destroy T cells. CBMΦ were less able to up-regulate CD80 and CD86 than PBMΦ (p < 0.05). Dexamethasone inhibited the up-regulation of CD80, CD86, and HLA-DR on PBMΦ and even more so on CBMΦ (p < 0.05 versus PBMΦ for CD80 and CD86). In the presence of dexamethasone, stimulation with αCD3 MAb enhanced cytotoxic functions of PMBMΦ and CBΜΦ with an increase in deleted T cells, a reduced fraction of enlarged T cells, and an inhibition of T cell CD28 up-regulation, which again were more pronounced with CBMΦ (p < 0.05 versus PBMΦ). In conclusion, neonatal MΦ are exquisitely sensitive to the inhibitory effects of dexamethasone on B7 expression. Although perhaps producing the desired therapeutic effect, dexamethasone may do so in newborns at the expense of a near complete paralysis of MΦ-dependent T cell function.
Similar content being viewed by others
Main
Preterm newborns have been exposed to dexamethasone for the prevention or treatment of bronchopulmonary dysplasia (BPD) for many years (1,2). Dexamethasone is a synthetic glucocorticoid that reduces the recruitment of inflammatory cells (3,4) and thereby is thought to inhibit the development of BPD. Its effects on other developing organs such as the CNS were only recognized much later. Because follow up-studies provided evidence of abnormal neurodevelopment, early postnatal use of dexamethasone is not recommended any more (2). Nevertheless, glucocorticoids continue to be given to pregnant women to accelerate fetal lung development.
Besides the endocrinium, the primary target of dexamethasone is the immune system, which is incompletely developed in neonates. Evidence that the immunosuppressive effects of dexamethasone are primarily mediated via an inhibition of cytokine production has been produced. Mononuclear cells from adults and neonates respond differently to treatment with dexamethasone. With respect to proinflammatory cytokines, cord blood cells show an increased sensitivity toward the inhibitory action of dexamethasone compared with cells from adult donors, resulting in a more pronounced inhibition of IL-1β, IL-6, tumor necrosis factor-α, IL-12, IL-2, and IL-3 production (5,6).
Dexamethasone may directly inhibit T cell proliferation (7), induce apoptosis (8), promote long-lasting changes in the T cell receptor vβ repertoire (9), or decrease the CD4/CD8 ratio in infants with BPD (10). Dexamethasone also influences gene expression of cytokines and various functions of antigen presenting cells (APC), including B cells, dendritic cells, and monocytes/macrophages (11–13).
T cell activation in the neonate is impaired (7). Beside intrinsic T cell deficiencies, their reduced capacity to become activated largely results from an impaired function of and interaction with APC (14–16). The ability of APC, including monocyte-derived macrophages (MΦ), to induce co-stimulatory signals in T cells by engaging their CD28 receptors is critically important for the T cell response. The lack of co-stimulation leads to anergy or apoptosis of antigen-reactive T cells (17,18). The CD28 ligands are expressed on MΦ and belong to the B7 receptor family. Beyond controlling T cell activation and cell death, B7 ligation influences T cell differentiation and cytokine production (19).
In humans, the B7 family consists of at least two molecules, B7-1 (CD80) and B7-2 (CD86), which belong to the immunoglobulin superfamily (20). Both bind to ligands on T cells, CD28, and CTLA4 (CD152) (21,22). The B7/CD28 family consists of additional receptors, each of which may promote activating or terminating signals (reviewed in 22).
CD86 (B7-2) is constitutively expressed on MΦ (21). The up-regulation of CD80 and CD86 occurs after contact with nominal antigen, IFN-γ (23), lipopolysaccharide (24), or mitogen-activated T cells (25) with different kinetics (26). In adult donors, glucocorticoids were found to inhibit the activation-induced expression of B7 receptors in MΦ (27).
Cord blood macrophages (CBMΦ) per se exhibit a reduced co-stimulatory potential: CD80 and CD86 expression and up-regulation are significantly inhibited in CBMΦ compared with MΦ from adult donors (16). Consistent with this observation, MΦ-dependent T cell activation is reduced in neonates, and CBMΦ preferentially deliver negative signals to T cells (16).
In view of the immaturity of the neonatal immune system, we were interested in investigating the capacity of neonatal MΦ to respond to dexamethasone. To our knowledge, its impact on neonatal MΦ and on MΦ-dependent T cell reactions has not yet been studied. Because mechanisms that control B7 receptors bear large consequence for the T cell response, we tested the hypothesis that CBMΦ would exhibit an increased sensitivity toward dexamethasone-induced inhibition of B7 expression and on T cell proliferation compared with PBMΦ.
METHODS
Patients.
The study protocol was approved by the Ethics Committee of the University of Tuebingen. All mothers gave written consent before they went into labor. Randomly selected, unrelated adult healthy volunteers donated blood and served as control subjects. All term neonates were delivered spontaneously and did not exhibit signs of infection, as defined by the clinical status, white blood cell count, and C-reactive protein. Mothers with amnion infections and prolonged labor were excluded. Umbilical cord blood was drawn from the fetal side of the placenta by puncture with a sterile needle, attached to a syringe without suction, and placed in heparin-coated tubes (100 IE/mL blood) immediately after ligation of the cord.
Cell cultures.
Peripheral blood (PBMCs) and cord blood mononuclear cells (CBMNC) were isolated by Ficoll-Hypaque (Pharmacia LKB, Uppsala, Sweden) density gradient centrifugation. Washed cells were resuspended in RPMI 1640 (Sigma Chemical Co., St. Louis, MO) that contained 10% FCS (Sigma Chemical Co.) and incubated at 37°C in a humidified incubator with a 5% CO2 atmosphere.
Preparation of mononuclear cell subsets.
Unseparated mononuclear cells were placed at 3 × 105 cells/0.1 mL in flat-bottom 96-well microtiter plates (Falcon, Bedford, MA). For separating macrophages from lymphocytes, cells were plated at 3 × 106 cells per 1.5 mL in 60 × 15-mm culture vessels (NoK4-3802-4; Becton Dickinson, Mountain View, CA) in the incubator and allowed to adhere for 60 min. Nonadherent cells were gently removed by repeatedly pipetting 500 μL of RPMI buffer into the cultures. Remaining adherent cells were washed thoroughly twice and used as a source of macrophages. Usually, 85% of adherent cells expressed CD14.
For further eliminating contaminating nonadherent macrophages, the procedure above described was repeated with the fraction of nonadherent cells. Usually, <1% CD14+ macrophages were found in the nonadherent fraction after this adherence cycle, as determined by FACS analysis.
Co-culture Experiments.
Macrophage-enriched adherent cells (1 × 105/0.05 mL) and macrophage-depleted nonadherent cells were mixed (2 × 105/0.05 mL). Nonadherent cell fractions were not pooled from different donors. For equalizing allogeneic effects, CBMΦ and PBMΦ of one adult donor were co-cultured with nonadherent cells of a second adult donor.
Flow cytometry.
A daily calibrated FACScan flow cytometer (Becton Dickinson) was used to perform phenotypic analysis. For preventing nonspecific binding, cells were incubated with 10% human serum on ice for 10 min before staining with FITC-, phycoerythrin-, or isotype-specific Ig-labeled MAb for 20 min over ice in the dark. MΦ were gated by forward, side scatter, and CD14 expression. For ensuring that larger cells were MΦ recently migrated and not lymphoblasts, a parallel analysis was performed with the MAb anti-CD3 (SK7). Dead lymphocytes were discriminated by propidium iodide (Molecular Probes, Eugene, OR; 5 μg/mL, 5 min). Propidium iodide–negative cells were counted and analyzed for expression of CD4 and CD8. T cell blasts were detected as CD4+ or CD8hi+ cells with enlarged size in the forward scatter as previously described (28).
Reagents.
Human recombinant IFN-γ, a potent inducer of CD80 and CD86 (15), was purchased from R&D Systems (Minneapolis, MN). Cell-permeable dibutyryl-cAMP, up-regulating the expression of CD86 but not of CD80 on MΦ (27), was obtained from Sigma Chemical Co.
Fresh dilutions of dexamethasone (Sigma Chemical Co.) in PBS solutions were prepared for each experiment and added at concentrations from 10−9 to 10−4 mol shortly before addition of B7-inducing substances. Serum concentrations to 10−5 mol may be reached pharmacologically (5). Cultures that were incubated in the absence of dexamethasone served as controls. The T cell mitogen anti-CD3 MAb (OKT3, 1 μg/mL) was purchased from Ortho Diagnostics (Raritan, NJ).
Antibodies to CD14 (MΦP9), CD80 (L307.4), CD86 (IT2.2), HLA-DR (L243), CD3 (SK7), CD4 (SK3), CD8 (SK1), and CD28 (L293) and Ig-matched controls (IgG1, IgG2b) were purchased from Becton Dickinson (Heidelberg, Germany). Tissue culture experiments were repeated at least three times. Results are expressed in mean ± SD.
Data display and statistical analysis.
Fluorescence intensities were determined, and the nonspecific background staining was subtracted. Statistical analysis was performed by using the decadic logarithm of the values of CD80, CD86, HLA-DR, and CD28. Using an ANOVA, we examined whether the variables were influenced by dexamethasone and age (both nominal effects). The variable “patient” (nominal) was nested under “age,” and the nested variables were modeled as a random effect. Furthermore, an interaction between “age” and “dexamethasone” was considered.
Using an analysis of covariance (ANCOVA), we examined whether the decadic logarithm of numbers of resting or blast-forming T cells were influenced by time, the origin of the blood, and dexamethasone. The variable “patient” was nested and modeled as a random effect. Furthermore, an interaction between “patient” and “day” was considered. Values of p < 0.05 were considered statistically significant. Statistical analysis was performed using the Sigmaplot 2000 software for Windows (SPSS, Chicago, IL).
RESULTS
Effect of dexamethasone on CD80, CD86, and HLA-DR expression.
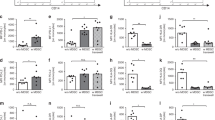
CBMΦ and PBMΦ were incubated for 48 h with and without various concentrations of dexamethasone. Spontaneous and IFN-γ–induced CD80 and CD86 expressions were determined. For examining whether the IFN-γ–induced effect was specific for CD80 and CD86, HLA-DR densities were also detected. Representative histograms for CD80 expression on PBMΦ (left) and CBMΦ (right) are depicted in Fig. 1.
CBMΦ spontaneously expressed CD80, CD86, and HLA-DR in lower densities than PBMΦ (p < 0.05; Fig. 2). Dexamethasone further decreased CD80 and CD86 expression in both groups (p < 0.05 versus unstimulated control) but did not influence the spontaneous HLA-DR expression. IFN-γ caused an up-regulation of all three receptors and was more pronounced on PBMΦ (p < 0.05 versus CBMΦ).
Inhibition of spontaneous and IFN-γ–induced expression of CD80 and CD86 by dexamethasone. PBMΦ (▪) and CBMΦ (□) were cultured for 48 h in the presence or absence of dexamethasone (concentrations as indicated in mol; +10−6 mol) and/or IFN-γ (500 U/mL). Dexamethasone was added 1 h before IFN-γ. Cells were harvested and phenotyped for CD80 (A), CD86 (B), or HLA-DR (C). Mean fluorescence intensities are depicted; five experiments are shown (mean ± SD); *p < 0.05 PBMΦ vs corresponding CBMΦ; +p < 0.05 dexamethasone-mediated inhibition across different dexamethasone concentrations PBMΦ vs CBMΦ.
Dexamethasone inhibited the IFN-γ–mediated up-regulation of CD80, CD86, and HLA-DR. On PBMΦ, this inhibitory effect on CD80 and in both MΦ populations the inhibition of HLA-DR expression were dose dependent. Dexamethasone-induced CD80- and CD86-related effects were stronger on CBMΦ (p < 0.01 versus PBMΦ); the inhibition of HLA-DR up-regulation did not differ significantly (p = 0.11). Survival, as detected by propidium iodide staining, was not affected by the drug (data not shown).
To investigate whether dexamethasone-mediated effects on CD80 and CD86 were restricted to IFN-γ, we used different B7 inducers (Fig. 3). Stimulation with cAMP did not affect CD80 expression (Fig. 3A) but resulted in increased CD86 receptor densities in both groups (Fig. 3B), with CBMΦ being less receptive (p < 0.05 versus PBMΦ). The addition of dexamethasone to this group inhibited CD86 up-regulation, again more pronounced on CBMΦ (p < 0.05 versus PBMΦ). The pattern seen with αCD3 was similar to IFN-γ stimulation with both: an impairment of CBMΦ to up-regulate CD80 and CD86 and a stronger inhibitory effect of dexamethasone (p < 0.05 versus PBMΦ).
Dexamethasone-induced inhibition of cAMP- and αCD3-induced B7 expression. Mononuclear cells (2 × 105/0.1 mL) from healthy adults (▪) and cord blood (□) were cultured for 48 h alone or in the presence of cAMP (10−3 mol) or αCD3 (1 μg/mL). Three groups received dexamethasone (10−6 mol). Cells were harvested and phenotyped in triplicate for the mean fluorescence expression of CD80 (A) or CD86 (B). Five experiments are shown (mean ± SD). *p < 0.05 PBMΦ vs corresponding CBMΦ; +p < 0.05 dexamethasone-mediated inhibition PBMΦ vs CBMΦ.
Effect of dexamethasone on MΦ-dependent αCD3-mediated T cell reactions.
In the presence or absence of dexamethasone, mononuclear cells from adults (PBMNC) or cord blood (CBMNC) were incubated with αCD3. The frequencies of enlarged T cells (Fig. 4A) as a parameter of T cell activation, and the absolute numbers of viable T cells, a parameter of T cell survival (Fig. 4B), were assayed daily.
Dexamethasone inhibits αCD3-mediated T cell blast transformation and promotes cell death. Unseparated PBMNC of healthy adults (circles) and cord blood (triangles), each containing comparable amounts of MΦ, were stimulated with αCD3 (1 μg/mL). Dexamethasone (10−6 mol; open symbols) was added before αCD3. Cells were counted and phenotyped for CD4 and CD8 expression daily. T cell blasts (A) and remaining T cells (B) were detected. Three experiments are shown (mean ± SD); *p < 0.05 vs αCD3-treated PBMNC; +p < 0.05 dexamethasone-mediated effect CBMNC vs PBMNC.
The fraction of enlarged T cells was increased in PBMNC (p < 0.05 after 48 and 72 h versus CBMNC; Fig. 4A). In contrast, the fraction of T lymphocytes that were deleted before cells had a chance to divide was higher in CBMNC (p < 0.05 versus PBMNC after 72 h).
Dexamethasone enhanced the fraction of initially deleted T cells in cord blood (p < 0.05 versus αCD3 and versus PBMNC) and inhibited T cell blast transformation in both groups (p < 0.05) but to a higher extent in CBMNC (p < 0.05 versus PBMNC after 72 h), consistent with the finding that the number of remaining T cells in this group was constantly decreasing.
For eliminating innate differences between neonatal and adult T cells, in particular their potentially different sensitivity toward dexamethasone, purified PBMΦ of one healthy adult donor, or CBMΦ, were co-cultured with MΦ-depleted nonadherent mononuclear cells of a second, unrelated healthy adult donor as a source of enriched T cells (Fig. 5).
CBMΦ are more sensitive toward dexamethasone-mediated inhibition of αCD3-mediated T cell activation than PBMΦ. MΦ-depleted PBMNC (2 × 105) from one healthy adult donor were co-cultured with PBMΦ (1 × 105) from a second adult donor (circles) or 1 × 105 CBMΦ (triangles) and αCD3 (1 μg/mL) was added. Two groups received dexamethasone (10−6 mol) 2 h before addition of αCD3 (open symbols). Samples were taken daily, counted, and phenotyped for CD4 and CD8 expression. T cell blasts (A) and remaining T cells (B) were depicted. No differences were seen in the nonstimulated groups (data not shown). Four experiments are shown (mean ± SD); *p < 0.05 vs αCD3-treated group with PBMΦ; +p < 0.05 dexamethasone-mediated effect CBMΦ vs PBMΦ.
CBMΦ diminished the fraction of proliferating T cells (p < 0.05 versus PBMΦ after 48 and 72 h; Fig. 5A) and enhanced the αCD3-mediated deletion of adult T cells (p < 0.05 versus PBMΦ after 72 h; Fig. 5B). Treatment with dexamethasone essentially showed findings identical to those depicted in Fig. 4. In the presence of CBMΦ, we found a significant impact on the decrease of remaining T cells and an almost abolished blast transformation (p < 0.05 versus PBMΦ after 48 and 72 h). Neither MΦ-depleted enriched T cells nor MΦ-enriched adherent cells showed significant proliferation or deletion in the presence of αCD3 (1 μg/mL; data not shown). In the described interval, we found no differences with regard to proliferation between PBMΦ that were co-cultured with autologous or allogeneic T cells (data not shown). Co-incubation of lower numbers of MΦ (5 × 10−4 MΦ) with T cells had less effect in both cord and peripheral blood (data not shown), indicating the importance of the local tissue environment and MΦ:T cell ratio in vivo.
In the αCD3-mediated reaction, engagement of B7 receptors with corresponding receptors on T cells leads to CD28 up-regulation on T cells, which undergo blast transformation (29,30). Using CBMΦ as a source of co-stimulatory receptors, αCD3-mediated CD28 up-regulation on T cells from healthy adult donors was impaired (p < 0.05 versus PBMΦ). Dexamethasone inhibited this αCD3-mediated CD28 up-regulation in both groups but significantly more so in the presence of CBMΦ (p < 0.05 versus PBMΦ; Table 1), further indicating the T cell inhibiting impact of the drug to be mediated via co-stimulatory molecules on APC.
DISCUSSION
Our data identify the CD80 and CD86 receptors as targets of dexamethasone-induced immune suppression and show that neonatal MΦ exhibit an increased sensitivity toward this drug-induced receptor inhibition. Functionally, this negatively influences the MΦ-dependent T cell activation (Figs. 4 and 5, Table 1). In addition and in contrast to PBMΦ, CD86 expression on neonatal MΦ is partially inhibited by the drug (Fig. 2B). Effects of dexamethasone on MΦ are not restricted to CD80 and CD86, because HLA-DR expression is inhibited as well (Fig. 2B). Compared with MΦ from adults, neonatal MΦ already show a reduced potential to up-regulate CD80, CD86, and HLA-DR (16) (Figs. 2 and 3). Thereby, MΦ-dependent T cell proliferation is inhibited in cord blood, and activation-induced cell death is promoted (Figs. 4 and 5).
MΦ possess the capacity to regulate the T cell response positively and negatively. We distinguished two principal cytokine-induced MΦ subsets. One, referred to as cytotoxic MΦ (Mc) (31), lacks B7 expression (32) and is induced by IL-10 (33). The second subset, referred to as helper MΦ (Mh) (31), is induced by IFN-γ and expresses CD80, CD86, or both. Disturbances in the Mh/Mc balance have been reported in various diseases (34–38). MΦ that express B7 family molecules induce neither T cell anergy nor T cell destruction by apoptosis, because both reactions are blocked in the presence of co-stimulation (28–30,39–41). MΦ that lack CD80 and CD86 expression are incapable of preventing the induction of anergy or apoptosis in conjugated T cells and act as negative immune regulators (42–44). In addition, MΦ have the capacity to actively destroy T cells that they target for conjugate formation (44). MΦ may express CD95 ligand in high concentration, engaging the T cell CD95 receptor in the apoptotic destruction of the T cell (45).
The Mh/Mc balance reveals itself in the polyclonal αCD3-mediated stimulation of T cells in our experimental setting: T cell reactivity depends on the number of MΦ and their capacity to up-regulate co-stimulatory molecules (28,30). When exposed to αCD3 MAb, T cells mount a biphasic immune response, characterized by an initial decline in their number and a subsequent clonal expansion (Figs. 4 and 5). Only the fraction of T cells that manages to block apoptosis by engagement of CD28 co-stimulatory molecules gets properly activated by αCD3 MAb (39) (Table 1). Other T cells remain anergic or become deleted (39).
The presence of neonatal MΦ, which were impaired to up-regulate CD80 and CD86 after challenge with αCD3 MAb (16) (Fig. 3), led to a strong decline in remaining T cells and a reduction in proliferating cells (Figs. 4 and 5). Enhancing B7 expression either via exchange of a MΦ type (Fig. 5) or by addition of IFN-γ, was associated with increased T cell blast formation and a decline in the fraction of deleted T cells (25,30,39). These results underscore the observation that the neonatal T cell response can be turned on to almost adult-like levels by immunocompetent APC (14,16).
Deficiencies in IFN-γ production and effect by neonatal monocytes, which partially are attributed to their immaturity (46), are well documented (47). Marodi (48) identified a deficient cytokine receptor signaling pathway via signal transducer and activator of transcription-1 phosphorylation in response to IFN-γ, which may help to explain functional consequences. Reduced basal and IFN-γ–induced HLA-DR expression and up-regulation on CBMΦ underscore earlier reports (47,49–51). Dexamethasone additionally inhibits IFN-γ transcription (52). In accordance with our results, dexamethasone inhibits IFN-γ–induced HLA-DR expression on human monocytic cell lines (50); however, in contrast to CD80 and CD86, our results suggest no significant differences in the dexamethasone-mediated inhibition of HLA-DR up-regulation between PBMΦ and CBMΦ (Fig. 2C). Whether the drug affects the HLA-DR–mediated antigen-presenting capacity of CBMΦ in particular remains the subject of further investigation.
Dexamethasone inhibited the activation-induced up-regulation of CD86 on CBMΦ and to a lesser extent on PBMΦ (Fig. 3). In contrast to Girndt (27), who found no drug-related influence on the CD86 regulation in MΦ from adults, we used unpurified mononuclear cells for stimulation with IFN-γ and cAMP, suggesting additional indirect effects of dexamethasone, e.g. via T cells.
Although we tried to minimize potentially different effects of dexamethasone on neonatal versus adult T cells by co-culture, our experimental setup neither excludes drug-related effects on αCD3-stimulated T cells, which may influence MΦ secondarily, nor rules out allogeneic factors that may influence the αCD3 reaction in long-term cultures. Memory T cells, which are characterized by the membrane determinant CD45RO, are virtually absent in cord blood (7) and might account for the increased dexamethasone sensitivity of CBMNC. However, addition of identical amounts of CD45RO cells by co-incubating T cells of adult donors with CBMΦ or PBMΦ (Fig. 5) indicates a drug-mediated effect on MΦ.
The effects of dexamethasone on CD80 receptor inhibition in adults were found to be transmitted via the cytoplasmic glucocorticoid receptor, because it could be abrogated by the addition of the glucocorticoid receptor antagonist RU38486 (27). The CD80 up-regulation was similarly inhibited by equipotent doses of hydrocortisone and prednisolone (27). Compared with adults, neonatal adrenals are functionally immature, as reflected by decreased neonatal plasma cortisol concentrations (53) and a decreased density of glucocorticoid receptors in the hippocampus (54). The increased sensitivity of CBMΦ to dexamethasone therefore may reflect an immunologic compensation for the low levels of glucocorticoids in newborn plasma, so the neonatal immune system may be functionally balanced in vivo.
Here we confirm earlier observations (16) that the newborn macrophage system emphasizes negative rather than positive immune regulation, which may be prudent because in establishing an immune repertoire, the newborn must guard him- or herself most diligently against autoimmune reactions. Our data show that although dexamethasone tilts the Mh/Mc balance of both adult and neonatal MΦ toward Mc dominance, neonatal MΦ are significantly more sensitive to this effect of dexamethasone. The drug nearly abrogates the expression of co-stimulatory molecules and strongly enforces Mc activities. Therefore, although the neonatal lung may well respond to dexamethasone, the price that the neonate pays in excessively suppressed immune function may prove prohibitive.
Abbreviations
- APC:
-
antigen presenting cell
- BPD:
-
bronchopulmonary dysplasia
- αCD3 Mab:
-
anti-CD3 monoclonal antibody
- CBMΦ:
-
cord blood monocyte-derived macrophages
- CBMNC:
-
cord blood mononuclear cells
- IFN-γ:
-
interferon-γ
- MΦ:
-
monocyte-derived macrophages
- PBMΦ:
-
peripheral blood monocyte-derived macrophages
- PBMNC:
-
peripheral blood mononuclear cells
References
Tsukahara H, Watanabe Y, Yasutomi M, Kobata R, Tamura S, Kimura K, Hiraoka M, Mayumi M 1999 Early (4–7 days of age) dexamethasone therapy for prevention of chronic lung disease in preterm infants. Biol Neonate 76: 283–290
Grier DG, Halliday HL 2003 Corticosteroids in the prevention and management of bronchopulmonary dysplasia. Semin Neonatol 8: 83–91
Li YH, Brauner A, Jonsson B, Van-der-Ploeg I, Soder O, Holst M, Jensen JS, Lagercrantz H, Tullus K 2001 Inhibition of macrophage proinflammatory cytokine expression by steroids and recombinant IL-10. Biol Neonate 80: 124–132
Papoff P, Christensen RD, Calhoun DA, Juul SE 2001 Granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor and neutrophils in the bronchoalveolar lavage fluid of premature infants with respiratory distress syndrome. Biol Neonate 80: 133–141
Bessler H, Mendel C, Straussberg R, Gurary N, Aloni D, Sirota L 1999 Effects of dexamethasone on IL-1β, IL-6, and TNF-α production by mononuclear cells of newborns and adults. Biol Neonate 75: 225–233
Bessler H, Kagazanov S, Punsky I, Sirota L 2001 Effect of Dexamethasone on IL-10 and IL-12p40 production in newborns and adults. Biol Neonate 80: 262–266
Aggarwal S, Gupta A, Nagata S, Gupta S 1997 Programmed cell death (apoptosis) in cord blood lymphocytes. J Clin Immunol 17: 63–73
Kavelaars A, Zijlstra J, Bakker JM, Van Rees EP, Visser GH, Zegers BJ, Heijnen CJ 1995 Increased dexamethasone sensitivity of neonatal leukocytes: different mechanism of glucocorticoid inhibition of T cell proliferation of adult and neonatal cells. Eur J Immunol 25: 1346–1351
Bakker JM, Kavelaars A, Kamphuis PJ, Zijlstra J, van Bel F, Heijnen CJ 2001 Neonatal dexamethasone treatment induces long-lasting changes in T-cell receptor vβ repertoire in rats. J Neuroimmunol 112: 47–54
Parimi PS, Birnrant DJ, Rao LV, Diaz G, Moore JJ 1999 Effect of dexamethasone on lymphocyte subpopulations in premature infants with bronchopulmonary dysplasia. J Perinatol 19: 347–351
van de Stolpe A, Caldenhoven E, Raaijmakers JA, van der Saag PT, Koenderman L 1993 Glucocorticoid-mediated repression of intracellular adhesion molecule-1 expression in human monocytic and bronchial epithelial cell lines. Am J Respir Cell Mol Biol 8: 340–347
Irakam A, Miskolci V, Vancurova I, Davidson D 2002 Dose-related inhibition of proinflammatory cytokine release from neutrophils of the newborn by dexamethasone, betamethasone, and hydrocortisone. Biol Neonate 82: 98–95
Orlikowsky T, Wang ZQ, Dudhane A, Dannecker GE, Niethammer D, Wormser GP, Hoffmann MK, Horowitz HW 2001 Dexamethasone inhibits CD4 T cell deletion mediated by macrophages from human immunodeficiency virus-infected individuals. J Infect Dis 184: 1328–1330
Ridge JP, Fuchs EJ, Matzinger P 1996 Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271: 1723–1726
Hunt DW, Huppertz HI, Jiang HJ, Petty RE 1994 Studies of human cord blood dendritic cells: evidence for functional immaturity. Blood 84: 4333–4343
Orlikowsky TW, Spring B, Dannecker GE, Niethammer D, Poets CF, Hoffmann MK 2003 Expression and regulation of B7 family molecules on macrophages (MPhi) in preterm and term neonatal cord blood and peripheral blood of adults. Cytometry 53B: 40–47
Schwartz RH 1992 Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71: 1065–1068
Janeway CA Jr, Bottomly K 1994 Signals and signs for lymphocyte responses. Cell 76: 275–285
Kuchroo VK, Das MP, Brown JA, Ranger AM, Zamvil SS, Sobel RA, Weiner HL, Nabavi N, Glimcher LH 1995 B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80: 707–718
Nabavi N, Freeman GJ, Gault A, Godfrey D, Nadler LM, Glimcher LH 1992 Signalling through the MHC class II cytoplasmic domain is required for antigen presentation and induces B7 expression. Nature 360: 266–268
Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R 1994 Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4-receptors. Immunity 1: 793–797
Salomon B, Bluestone JA 2001 Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 19: 225–252
Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C 1993 B70 antigen is a second ligand for CTLA-4 and CD28. Nature 366: 76–79
Schmittel A, Scheibenbogen C, Kielholz U 1995 Lipopolysaccharide effectively upregulates B7-1 (CD80) expression and costimulatory function of human monocytes. Scand J Immunol 42: 701–704
Orlikowsky T, Wang ZQ, Dudhane A, Horowitz HH, Conti B, Hoffmann MK 1997 Two distinct pathways of human macrophage differentiation are mediated by interferon-gamma and interleukin-10. Immunol 91: 104–108
Hathcock KS, Laszlo G, Pucillo C, Linsley PS, Hodes RJ 1994 Comparative analysis of B7-1 and B7-2 costimulatory ligands: expression and function. J Exp Med 180: 631–640
Girndt M, Sester U, Kaul H, Hünger F, Köhler H 1998 Glucocorticoids inhibit activation-dependent expression of costimulatory molecule B7-1 in human monocytes. Transplantation 66: 370–375
Orlikowsky T, Dannecker GE, Wang Z, Horowitz H, Niethammer D, Hoffmann MK 1999 Activation or destruction of T cells via macrophages. Pathobiology 67: 298–301
Linsley PS, Ledbetter JA 1993 The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol 11: 191–212
Wang ZQ, Orlikowsky T, Dudhane A, Trejo V, Hoffmann MK 1998 Macrophages may activate or destroy T cells with which they form antigen- or coreceptor-mediated cellular conjugates. Cell Immunol 189: 74–82
Kümmerle-Deschner JB, Hoffmann MK, Niethammer D, Dannecker GE 1998 Pediatric rheumatology: autoimmune mechanisms and therapeutic strategies. Immunol Today 19: 250–253
Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM 1993 IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the upregulation of B7 expression. J Immunol 151: 1224–1234
Wang ZQ, Bapat AS, Rayanade RJ, Dagtas AS, Hoffmann MK 2001 Interleukin-10 induces macrophage apoptosis and expression of CD16 (FcγRIII) whose engagement blocks the cell death programme and facilitates differentiation. Immunology 102: 331–337
Trinchieri G 1997 Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-gamma). Curr Opin Immunol 9: 17–23
Wilder RL, Elenkov IJ 1999 Hormonal regulation of tumor necrosis factor-alpha, interleukin-12 and interleukin-10 production by activated macrophages. A disease-modifying mechanism in rheumatoid arthritis and systemic lupus erythematosus?. Ann NY Acad Sci 876: 14–31
Kataoka Y, Iwasaki T, Kuroiwa T, Seto Y, Iwata N, Hashimoto N, Ogata A, Hamano T, Kakishita E 2001 The role of donor T cells for target organ injuries in acute and chronic graft-versus-host disease. Immunology 103: 310–318
Wang Z, Horowitz HW, Orlikowsky T, Hahn BI, Trejo V, Bapat AS, Mittler RS, Rayanade RJ, Yang SY, Hoffmann MK 1999 Polyspecific self-reactive antibodies in individuals infected with human immunodeficiency virus facilitate T cell deletion and inhibit costimulatory accessory cell function. J Infect Dis 180: 1072–1079
Wang ZQ, Horowitz HW, Orlikowsky T, Dudhane A, Weinstein A, Hoffmann MK 1998 Lymphocyte-reactive autoantibodies in human immunodeficiency virus type 1-infected persons facilitate the deletion of CD8 T cells by macrophages. J Infect Dis 178: 404–412
Wang ZQ, Bapat AS, Trejo V, Orlikowsky T, Mittler RS, Hoffmann MK 1999 MHC class I molecules on CD4 T cells regulate receptor-mediated activation signals. Cell Immunol 193: 108–114
Sperling AI, Auger JA, Ehst BD, Rulifson IC, Thompson CB, Bluestone JA 1996 CD28/B7 interactions deliver a unique signal to naive T cells that regulates cell survival but not early proliferation. J Immunol 157: 3909–3917
Hurtado JC, Kim YJ, Kwon BS 1997 Signals through 4-1BB are costimulatory to previously activated splenic T cells and inhibit activation-induced cell death. J Immunol 158: 2600–2609
Orlikowsky T, Wang ZQ, Dudhane A, Horowitz HW, Riethmuller G, Hoffmann MK 1997 Cytotoxic monocytes in the blood of HIV type 1-infected subjects destroy targeted T cells in a CD95-dependent fashion. AIDS Res Hum Retroviruses 13: 953–960
Wang Z, Horowitz HW, Orlikowsky T, Hahn BI, Trejo V, Bapat AS, Mittler RS, Rayanade RJ, Yang SY, Hoffmann MK 1999 Polyspecific self-reactive antibodies in individuals infected with human immunodeficiency virus facilitate T cell deletion and inhibit costimulatory accessory cell function. J Infect Dis 180: 1072–1079
Dudhane A, Conti B, Orlikowsky T, Wang ZQ, Mangla N, Gupta A, Wormser GP, Hoffmann MK 1996 Monocytes in HIV type 1-infected individuals lose expression of costimulatory B7 molecules and acquire cytotoxic activity. AIDS Res Hum Retroviruses 12: 885–892
Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC 1997 Human monocytic cells contain high levels of intracellular Fas ligand: rapid release following cellular activation. J Immunol 159: 1594–1598
Stiehm ER, Sztein MB, Steeg PS, Mann D, Newland C, Blaese M, Oppenheim JJ 1984 Deficient DR antigen expression on human cord blood monocytes: reversal with lymphokines. Clin Immunol Immunopathol 30: 430–436
Taylor S, Bryson YJ 1985 Impaired production of gamma-interferon by newborn cells in vitro is due to a functionally immature macrophage. J Immunol 134: 1493–1497
Marodi L 2002 Deficient interferon-gamma receptor-mediated signaling in neonatal macrophages. Acta Paediatr Suppl 91: 117–119
Birle A, Nebe TC, Gessler P 2003 Age-related low expression of HLA-DR molecules on monocytes of term and preterm newborns with and without signs of infection. J Perinatol 23: 294–299
Schwiebert LM, Schleimer RP, Radka SF, Ono SJ 1995 Modulation of MHC class II expression in human cells by dexamethasone. Cell Immunol 165: 12–19
Wakasugi N, Virelizier JL 1985 Defective IFN-γ production in the human neonate. I. Dysregulation rather than intrinsic abnormality. J Immunol 134: 167–171
Arya SK, Wong-Staal F, Gallo RC 1984 Dexamethasone-mediated inhibition of human T cell growth factor and gamma-interferon messenger RNA. J Immunol 133: 273–276
Munck A, Guyre PM 1990 Psychoneuroendocrinology, 2nd Ed. Acad Press, San Diego, pp 447–461
Rosenfeld P, Van Eekelen JA, Levine S, de Kloet ER 1993 Ontogeny of glucocorticoid receptors in the brain. Cell Mol Neurobiol 13: 261–277
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Orlikowsky, T., Dannecker, G., Spring, B. et al. Effect of Dexamethasone on B7 Regulation and T Cell Activation in Neonates and Adults. Pediatr Res 57, 656–661 (2005). https://doi.org/10.1203/01.PDR.0000156211.48307.F5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000156211.48307.F5