Abstract
Lower limits of protein needs in prematurely born neonates have not been adequately studied, yet providing protein in amounts maximizing accretion without excess is a goal in these infants' nutritional care. We hypothesized that with the use of amino acid oxidation methodology, it would be possible to define minimum protein requirement. Our objective was to investigate protein kinetics during short-term changes in protein intake by measurement of nitrogen balance and amino acid flux and oxidation using [15N]glycine, [13C]phenylalanine, and [13C]leucine tracers. Protein kinetics were examined in 21 preterm infants (gestational age: 29 ± 3 wk; birth weight: 1091 ± 324 g) at five protein intakes (1.0, 1.5, 2.0, 2.5, and 3.0 g·kg−1·d−1) with 1 d of adaptation to the test intakes. From nitrogen balance data, a protein need of 0.74 g·kg−1·−1 was estimated to achieve zero balance. For all three amino acids, flux and oxidation estimates were not different across protein intakes. Whole-body protein synthesis and breakdown estimates from [15N]ammonia data were 14.6 ± 3.4 and 14.4 ± 4.1 g·kg−1·d−1, respectively. Glycine flux (680 ± 168 μmol·kg−1·h−1) was greater than leucine flux (323 ± 115 μmol·kg−1·h−1), which was greater than phenylalanine flux (84.3 ± 35.2 μmol·kg−1·h−1). Leucine oxidation (36.7 ± 15.6 μmol·kg−1·h−1) was also greater than phenylalanine oxidation (6.64 ± 4.41 μmol·kg−1·h−1). Infants in our study were able to adapt to short-term changes in protein intake with little consequence to the overall whole-body protein economy, as measured by the three test amino acids.
Similar content being viewed by others
Main
Human neonates, particularly those born prematurely, have limited nutritional reserves during a period of rapid growth (1). Physiologic immaturity and illness also make supplying the preterm neonate's nutritional requirements difficult (2). Therefore, a goal in the nutritional management of the neonate is to provide dietary protein in amounts that maximize synthesis and accretion without exceeding the capacity for removal of excess amino acids (3, 4).
Intrauterine accretion rates have been widely used as the basis of protein requirements in the neonate (4). A protein intake of 1.5 g·kg−1·d−1 is recommended in the preterm infant to prevent breakdown of endogenous tissue, with amounts increasing to 2.0-3.4 g·kg−1·d−1 to achieve intrauterine accretion once the infant is stable (4, 5). However, clinical status (i.e. fluid tolerance, sepsis, and respiratory insufficiency) often limits fluid volumes and amount of feeding possible (4). Therefore, defining the lower limit(s) of the protein needs of the premature is imperative as lower protein intakes are often an inevitable part of neonatal intensive care.
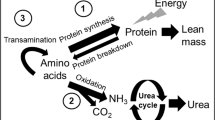
Most of our understanding of whole-body nitrogen metabolism comes from nitrogen balance studies. It gives no insight into the mechanisms of how the body's protein metabolism adapts to changes in nutrient intake over the short term. Furthermore, nitrogen balance studies have experimental design constraints because feeding low birth weight neonates low-protein formulas for an extended period of time would be unethical. Investigations in adults (6, 7) have shown with the use of amino acid oxidation methodology that the optimal requirement for certain indispensable amino acids may have been underestimated by the nitrogen balance technique. An advantage of the oxidation technique is that the amino acid metabolism has been shown to respond promptly (2-3 h) to a change in amino acid intake (8, 9); therefore, oxidation measurements provide information on short-term changes in protein metabolism without the ethical dilemma of infants' receiving a protein-deficient diet for an extended period of time (i.e. longer than 1 d). In light of these findings, it is necessary to reinvestigate the protein needs of the infant using this newer, more sensitive method. Application of these oxidation studies in the preterm infant has the potential to better define not only neonatal protein needs but also the lower limit of protein requirements.
A number of approaches, which use amino acids labeled with stable isotopes of carbon and nitrogen, to measure rates of whole-body nitrogen turnover, protein synthesis, and degradation have been developed and used in the neonate. The common amino acid tracers used in preterm neonatal flux and oxidation studies have been [15N]glycine (5, 10–13) and [13C]leucine (14–22) and, more recently, [5H2]phenylalanine (23, 24) and [13C]phenylalanine (25). Although these studies have provided valuable insight on various aspects of wholebody protein metabolism, as well as individual amino acid metabolism, information on the protein requirements of premature neonates has not been forthcoming in the flux and oxidation studies performed to date.
In adults (9, 26) and children (27), we have used the oxidation of the tracers L-[1-13C]phenylalanine, L-[1-13C]leucine, and L-[1-13C]lysine as markers of both amino acid and protein adequacy in diets when consumed at varying intakes of amino acids and protein. The amino acid oxidation technique is based on the principle that the oxidation of an indispensable amino acid is dependent on the dietary level of protein or of the most limiting amino acid, with the level of oxidation being proportional to the degree of deficiency (7). The technique is representative of the changes in protein synthesis when dietary intake of protein or the limiting amino acid is varied.
In studies in adults (7–9) and children (27), we have demonstrated a break point in label oxidation (13CO2 rate of appearance in breath), which reflected the requirement point. Only in an adult protein requirement study (26) did we adapt the subjects for more than a few hours to the change in intake. We did so to permit adaptation in the urea pool so as to be able to use nitrogen balance as an outcome variable, in addition to amino acid oxidation. We were not able to use any more than 12 h of adaptation to the new level of protein intake in the present study because it is not ethical to feed preterm intakes at protein intakes less than the 1.5 g·kg−1·d−1, which is the currently accepted minimal intake for protein in this group (4). Our ethics committee believed that 24 h below this level was reasonable, but no longer. In the present study, we expected to see a break point in phenylalanine or leucine oxidation. In addition, we hypothesized that changes in protein intake levels might be reflected in whole-body protein turnover, measured using the appearance of 15N-ammonia, arising from the 15N-glycine tracer. The excretion of urinary nitrogen was also measured to estimate nitrogen balance over the 24 h of the altered protein intake. On the basis of our earlier studies (7–9, 27), a break point in amino acid oxidation or a change in whole-body protein turnover would have been an indication of an adaptation of the infant's amino nitrogen metabolism in response to too low an intake in total protein. Finally, our previous nitrogen balance studies in preterm neonates had not gone low enough in the level of protein intake (12) to have any of the subjects in zero let alone negative nitrogen balance. The level of protein at which zero balance occurred was estimated by extrapolation backward, which is not necessarily valid. In the present study, by going as low as 1 g protein·kg−1·d−1, we expected to be able to determine directly and for the first time the level of protein needed to achieve nitrogen equilibrium. This we would define as the absolute minimum protein intake for the neonate, who in fact is expected to be net accreting nitrogen and protein (3, 4).
METHODS
Subjects.
Twenty-one prematurely born infants who were of gestational age ≤34 wk (28) and had been referred to the neonatal intensive care unit of The Hospital for Sick Children, Toronto, were studied. The infants were of appropriate weight for gestational age with birth weights ranging from 630 to 1820 g. All were healthy, growing, orally fed neonates, receiving approximately 460 kJ·kg−1·d−1 (110 kcal·kg−1·d−1) of standard low birth weight infant formula [Similac Special Care (SSC); Abbott-Ross Laboratories, Montreal, Canada]. The whey-predominant formula provided approximately 3 g protein·kg−1·d−1 (1.66 g protein/dL). The infants were fed by bottle or via nasogastric tube every 2 h. Any infants who had congenital anomalies or were receiving any drugs were excluded from the study. The experimental protocol and procedures were approved by the Human Subject Review Committee of The Hospital for Sick Children, and informed written consents were obtained from one or both of the infants' parents.
Experimental design.
Protein kinetics at varying protein intake were determined using nitrogen balance and stable isotope methodology using [15N]glycine, L-[1-13C]phenylalanine, and L-[1-13C]leucine as tracers. A baseline study was carried out on all infants who were receiving the SSC formula for at least 3 d. In this study, protein kinetics were studied using [15N]glycine (n = 21) and either [13C]phenylalanine (n = 9) or [13C]leucine (n = 5). Two days later, infants were allocated to one of four low-protein formulas for a period of 24 h. These formulas provided the same amount of energy and micronutrients as SSC, but the level of protein was modified to 0.55, 0.80, 1.11, and 1.38 g protein/dL to provide approximately 1.0, 1.5, 2.0, and 2.5 g·kg−1·d−1. These experimental formulas were made specifically for this study by Wyeth-Ayerst Laboratories (Philadelphia, PA). The formulas were kept isocaloric by adjusting carbohydrate and fat in equal proportions. During this time, an amino acid kinetic study was carried out using the same two labeled amino acids that the infants received during the baseline study. After this study, the infants were returned to their standard formula regimen for at least 3 d. In available infants, this process was again repeated at different protein intakes. In total, 56, 26, and 13 infusion studies using [15N]glycine, [13C]phenylalanine, and [13C]leucine, respectively, were carried out at various dietary protein intakes. Amount of formulas ingested was closely monitored and recorded throughout the period. Infants were weighed daily, and their growth was monitored (i.e. length and head circumference).
Study protocol.
Three amino acid tracers were used: [15N] glycine, L-[l-13C]phenylalanine, and L-[l-13C]leucine (Merck Frosst, Montreal, Canada). Enrichment was verified by gas chromatography-mass spectrometry. Quality-control tests performed on the amino acid isotopes by Merck Frosst included optical rotation tests, thin-layer chromatography, and nuclear magnetic resonance, which confirmed chemical and optical purity (i.e. >98% l-13C for both phenylalanine and leucine isotopes). Separate solutions (10 g/L) of each tracer were prepared with sterile water under a laminar flow hood. The solutions were sterilized by passage through a 0.22-μm filter (Millipore Corp., Bedford, MA) under laminar flow and were put into single-dose vials. Each batch was determined to be sterile and pyrogen-free.
The oral administration of the amino acid tracer doses simulated the primed, constant isotope infusion technique (25). Primed, constant infusions of [15N]glycine (7 μmol/kg and 7 μmol/kg−1·h−1, prime and infusion doses, respectively) and [13C]phenylalanine (29 μmol/kg and 29 μmol·kg−1·h−1) or [13C]leucine (38 μmol·kg and 19 μmol/kg−1·h−1) were carried out in each infant for 16-24 h. An aliquot of the combined tracer solution was administered directly into the feeding tube or bottle nipple immediately before each 2-h feeding starting at 1800 h. The priming doses were given with the first aliquot.
A 3-h baseline sample of urine was collected by a condom collector before the start of the tracer infusion. Urine samples were then collected every 2 h for the duration of the infusion period immediately before feeding of the infant. The urine was stored at −20°C until analysis.
Breath samples were collected before and then every 2 h for the duration of the infusion period. Similar to urine sampling, breath was collected immediately before each feeding. To sample breath, a transparent thermoplastic hood was placed over the head and upper body of the infant while the infant rested in his or her crib or isolette (29). Air was drawn through the hood at a rate of approximately 1.5 L·kg−1·min−1 as measured by a mass flow meter (model 5810, 0-10 L/min; Brooks Instrument Division, Emerson Electric Company, Stouffville, Canada) and into an indirect calorimeter to measure production of carbon dioxide (VCO2). Carbon dioxide in the hood was maintained at approximately 0.5% by measuring CO2 concentration with a LB-2 Medical Gas Analyzer (SensorMedics; Beckman, Palo Alto, CA, U.S.A.) and adjusting the air flow rate through the hood. Samples of breath were collected from air exiting the CO2 analyzer at 500 mL/min for 10 min, which was then bubbled through 10 mL of a 1-M NaOH solution to trap the CO2 (30). The resulting NaHCO3 solution was stored at −20°C in evacuated glass tubes (Vacutainer #6441, Becton Dickinson Inc., Mississauga, Canada) until analysis for 13CO2 enrichment.
Sample analyses.
Urine samples (250 μL) were deproteinized with trichloroacetic acid and the free amino acids were isolated and derivatized to their N, O-heptafluorobutyryl isobutyl esters as previously described (25, 31). Isotopic enrichment of glycine, phenylalanine, and leucine was measured on a gas chromatograph-quadruple mass spectrometer (models 5840A/5985B; Hewlett-Packard, Mississauga, Ontario) (25). The enrichment, in molecule percent excess, was calculated by using the equation described by Matthews et al. (32). Isotopic steady state in the metabolic pool was represented by plateau in enrichment of urinary amino acids. Attainment of plateau, which was reached during the last 10-12 h of the infusion and was represented by at least three data points, was defined by consideration of the slope and SD of the points on the plotted enrichment curve. Glycine, phenylalanine, and leucine fluxes (μmol·kg−1·h−1) were measured from the dilution of the infused tracer in the metabolic pool at isotopic steady state, as previously described (8, 16, 25, 32).
The enrichment of 13C in breath CO2 was measured on a dual inlet isotope ratio mass spectrometer (VG Micromass 602D, Cheshire, England). Mass spectrometry analysis was performed using techniques described previously (30). Breath enrichments from baseline samples and from those taken during the isotope infusion were expressed as atoms percent excess 13CO2 over a reference standard of compressed CO2 gas. Isotopic steady state was represented by plateau in enrichment of CO2 and, as with urine, was composed of at least three data points taken toward the end of the infusion. The rate of phenylalanine or leucine oxidation (μmol·kg−1·h−1) was determined from the rate of excretion of 13CO2 (F13CO2) and from the enrichment of urinary phenylalanine and leucine at isotopic steady state as previously described (29). The oxidation rates were corrected because in a population of infants studied in the fed state, 80% of the 13CO2 was expired and the remainder was retained in the body because of bicarbonate fixation (33).
Amino nitrogen metabolism (i.e. synthesis and breakdown) was studied using the Picou and Taylor-Roberts model (34). 15N enrichment of urinary urea and ammonia was measured on a dual inlet isotope ratio mass spectrometer (VG Micromass 602D) using techniques described previously (12). Only [15N]ammonia data were used to determine flux and wholebody protein synthesis, and breakdown as isotopic steady state was not achieved in [15N]urea during the isotope infusion period of 24 h.
Formulas and urine collected during the infusion period were analyzed for total nitrogen by a micro-Kjeldahl procedure (29, 35). Energy content of the formulas was measured by oxygen bomb calorimetry. Apparent nitrogen balance (mg N·kg−1·d−1) was calculated from the nitrogen intake minus urinary nitrogen excretion during the study period. Because the present experiment was short term as a result of ethical considerations, fecal samples collected after 1 d of feeding a new diet would not have accurately represented the correct fecal excretion for that diet. Therefore, we used literature values (36) for fecal excretion in similar infants fed similar formulas. In that research, 3-d nitrogen balance studies were completed and fecal nitrogen losses were fairly constant at 19% of intake.
Statistical analysis.
Results are expressed as mean ± SD. Statistical analyses were performed on primary and derived values. ANOVA was used to compare subject characteristics and verify similar energy intakes in the different protein intake groups. Regression analysis was performed on nitrogen balance data, and confidence intervals (±95%) were determined for the regression line. The amino acid kinetic and oxidation data were analyzed as the difference within subject between the test protein intake compared with the SSC formula. Because there was significant variation in the actual protein intakes consumed by the neonates, individual protein intakes versus flux and oxidation values were plotted and regression analysis was performed for the various tracers used (37). A p ≤ 0.05 was considered significant. Statistical analyses were performed using SAS (38).
RESULTS
Clinical characteristics of the all of the neonates (n = 21) and the subgroups of neonates who received either the [13C]phenylalanine (n = 9) or the [13C]leucine (n ± 5) infusions along with the [15N]glycine (n = 21) infusion are shown in Table 1. The infants' gestational age at birth and birth weights ranged from 24 to 34 wk and 630 to 1820 g, respectively. All were appropriate for gestational age. The mean age of these infants at the time of the study was 55 ± 27 d or a postconceptional age of 36 ± 3 wk. The mean study weight of the infants of 1965 ± 391 g suggests that they were gaining weight appropriately since birth. No significant differences in birth and study weights, gestational and postconceptional ages, and sex were found among the three groups of neonates who were receiving the various isotopes.
Although all infants (n = 21) were studied at the 3.0 g·kg−1·d−1 protein level (i.e. SSC) with the glycine tracer, the number of studies performed at the other protein levels using the three tracers varied as a result of the availability of each neonate for additional studies. In total, 56 [15N]glycine, 26 [13C]phenylalanine, and 13 [13C]leucine infusions are reported in 21, nine, and five neonates, respectively.
The energy and nutrient intakes of the neonates in their assigned formula groups are summarized in Table 2. Energy intake was very close to the goal of 460 kJ·kg−1·d−1. Actual protein intakes at various levels studied were slightly lower than the targets of 1.0, 1.5, 2.0, 2.5, and 3.0 g·kg−1·d−1. In both the phenylalanine and leucine subgroups, protein and energy intakes were essentially the same as in the total group of neonates (data not shown). As expected, total intakes of nitrogen, glycine, phenylalanine, and leucine also increased as protein intakes were raised from 1.0 to 3.0 g·kg−1·d−1. However, as urinary nitrogen excretion was unchanged over the five protein intakes, a significant decrease in both apparent and corrected nitrogen balance resulted as daily protein intake was reduced (Table 2).
Variation in daily protein and energy intake was apparent within each formula group as a result of the neonates' receiving more or less of their assigned daily intake. This is not uncommon in studies of the preterm infant because of ethical considerations and the clinical nature of the experiments. Therefore, data for nitrogen balance, [15N]ammonia and amino acid fluxes, and amino acid oxidation are reported as individual rather than group data to represent actual protein intakes in Figs. 1,2, 3, and 4, respectively.
Nitrogen balance in preterm neonates over a range of protein intakes. □, individual measurement; dotted line is extrapolated from the thick solid regression line and crosses zero nitrogen balance at a protein intake of 0.74 g·kg−1·d−1. Equation of this line is y = 123.6x − 68.3, r2 = 0.947, p < 0.0001. Equations for the upper and lower 95% confidence intervals are y = 131.6x − 51.3 and y = 115.6x − 85.4, respectively, and are represented by the thin solid lines.
Nitrogen balance, corrected for fecal losses, versus protein intake in 56 studies is illustrated in Fig. 1. Extrapolating the linear regression data, a value of zero nitrogen balance would correspond to a mean protein intake of 0.55 g·kg−1·d−1 or 0.74 g·kg−1·d−1 when using the lower confidence interval as a measure of minimum protein intake. [15N]Ammonia flux is shown in Fig. 2. Regression analysis of these data produced a line with a correlation that was very low (r2 = 0.18) and a high error; therefore, we pooled the individual data across intakes to give a mean flux for [15N]ammonia of 2.47 ± 0.67 g N·kg−1·d−1. Calculated means for protein synthesis and breakdown were 14.6 ± 3.4 and 14.4 ± 4.1 g protein·kg−1·d−1, respectively.
Amino acid flux (μmol·kg−1·h−1) across protein intakes is illustrated in Fig. 3. For all three amino acids, flux was unaffected by protein intake (slope of regression line not different from zero). Overall mean glycine flux (680 ± 168) was greater than leucine flux (323 ± 115), which was, in turn, greater than phenylalanine flux (84.3 ± 35.2).
Oxidation values obtained from the phenylalanine and leucine tracers are shown in Fig. 4. Leucine oxidation was greater and more variable than phenylalanine oxidation with a mean (±SD) of 36.7 ± 15.6 μmol·kg−1·h−1 compared with 6.64 ± 4.41 μmol·kg−1·h−1, respectively. Oxidation values were not different across protein intakes.
DISCUSSION
Short-term decreases in dietary intake are an inevitable consequence in the complex care of preterm infants. In feeding these infants, the amount of protein received is directly related to energy intake; therefore, the effects of changes in protein intake alone are not well known. Therefore, this study was designed to examine protein kinetics in preterm infants as momentary protein intakes (i.e. short-term duration of 1 d) were lowered and energy intake remained constant. Furthermore, by using the stable isotope technique of amino acid oxidation, we aimed to detect the lower limit of adaptation of protein metabolism of the neonate to lowered levels of protein intake and, hence, determine lower limits of protein and nitrogen needs of the preterm human neonate. This technique has been applied successfully in the past to determine both individual amino acid (9) and protein requirements (26) in human adults. The amino acids chosen for this study were two commonly used tracers, L-[13C]phenylalanine and L-[13C]leucine. In addition, whole-body nitrogen metabolism was studied using [15N]glycine and nitrogen balance in the neonates was measured.
As expected, nitrogen balance decreased as protein intake was lowered; however, in only one of 56 studies was nitrogen balance negative. When the lower 95% confidence interval was used as a measure of minimum protein need, a value of 0.74 g·kg−1·d−1 was obtained for zero nitrogen balance. This value is lower than the previously reported mean value of 1.1 g protein·kg−1·d−1 (4, 5), which was based on a smaller sample size (n = 10) and a lowest protein intake of 1.5 g·kg−1·d−1.
Using [15N]ammonia data to determine protein synthesis and breakdown rates, we obtained values of 14.6 and 14.4 g·kg−1·d−1, respectively, which were similar to whole-body protein turnover rates in preterm infants reported by others, ranging from 10 to 14 g·kg−1·d−1, when using [15N]glycine as a tracer and [15N]ammonia and [15N]urea as end products (10, 11, 21). Calculation of whole-body protein turnover from leucine flux, assuming fetal body composition to contain 8.2% leucine (626 μmol/g), gave a value of 12.4 g·kg−1·d−1, which was similar to values obtained by others of between 10 and 15 g·kg−1·d−1 (15, 21, 25).
Flux and oxidation values obtained for the individual amino acid tracers were comparable to those reported in the literature for enterally fed preterm neonates. Overall glycine flux (680 μmol·kg−1·h−1) was similar to that reported previously by our group (25) of 560 μmol·kg−1·h−1 in a group of infants receiving 2.7 g protein·kg−1·d−1. Similarly, a leucine flux of 323 μmol·kg−1·h−1 was comparable to those reported by others (ranging from 260 to 380 μmol·kg−1·h−1) (14, 16, 19, 21, 25). Our leucine oxidation rate of 37 μmol·kg−1·h−1 was the same as reported by Denne et al. (18) and comparable to the estimates ranging from 40 to 55 μmol·kg−1·h−1 reported by Van Goudoever et al. (20, 21), who used α-KICA enrichment in estimating leucine kinetics. Both leucine flux and oxidation values were lower than those reported by van Toledo-Eppinga et al. (22); however, their preterm infants also had intrauterine growth retardation. The value obtained for phenylalanine flux (84.3 μmol·kg−1·h−1) was higher than previously reported by our group (56 μmol·kg−1·h−1) (25). Overall, phenylalanine oxidation was somewhat lower at 6.6 μmol·kg−1·h−1 than previously reported values of approximately 10 μmol·kg−1·h−1 (23, 24). However, in these other studies, [5H2]phenylalanine was used as the tracer to estimate hydroxylation rates (23, 24).
Neither flux nor oxidation rates of the amino acids changed over a range of protein intakes from approximately 0.8 to 3.5 g·kg−1·d−1. Lack of change in oxidation of the amino acids indicates that the intake of these amino acids had not exceeded their requirement. Increased intake of amino acid below requirement does not result in changes in amino acid oxidation, whereas increasing intake of an amino acid above requirement break point results in linear increases in oxidation (7, 8). This is also supported by the linear increase in nitrogen balance. Taken together, these data mean that protein intake to maximize nitrogen retention had not been achieved in these infants. Indeed, Synderman et al. (39) showed that nitrogen accretion was linear in premature neonates up to protein intakes of 9 g·kg−1·d−1.
One unique aspect of our study was that the infants had not been previously adapted to the test protein levels, because knowingly feeding infants inadequate protein intakes for any significant time period was considered unethical, and before the present research, we did not know the minimum protein intake that would result in negative nitrogen balance. In most of our amino acid and protein requirement experiments, subjects consumed a specific intake of protein before study. Using this approach, we have shown in studies of amino acid requirement in adult humans (7, 26) and infants (40) and in baby pigs (41) that previous adaptation to a test amino acid was not necessary when previous protein and amino acid intake was fixed for all treatments. Similarly, in studies of growing piglets, which used the indicator oxidation technique to determine protein requirement, adaptation was not needed (42). Conversely in nitrogen balance studies in adults, 7-10 d of adaptation are needed to permit full equilibration of the body urea pool and hence urinary nitrogen excretion (43). Interestingly, in the present study, there were no changes in urinary nitrogen excretion (Table 2) across the range of protein intakes. This may mean that these neonates were already in a state of maximal nitrogen conservation and therefore each additional increment of protein consumed was retained with high efficiency. Conversely, it may be that insufficient time was permitted to allow for adaptation to take place. Against this interpretation is the strong linear correlation (r2 = 0.947) between protein intake and nitrogen balance shown in Fig. 1. There is no evidence of nitrogen being retained in the body urea pool, in our earlier studies, as evidenced by urea averaging 54% of total urinary nitrogen (5).
In this study, we found that the minimum protein intake of premature infants, based on zero nitrogen balance, corresponds to a mean of 0.55 g protein·kg−1·d−1 or 0.74 g protein·kg−1·d−1 when using the lower confidence interval as a measure of minimum protein intake for the population. This study provides new information because of the large sample size (n = 56) and the control of energy intake. With this sample size, it is possible not only to define the mean for the population (the so-called estimated average requirement) but also to determine variance and hence establish a value to cover 95% of the population, thus being comparable to the recommended dietary allowance for the population. The mean value is approximately half the value we reported earlier of 1.1 g·kg−1·d−1 with a smaller sample size (n = 10) and backward extrapolation of the regression to zero nitrogen balance. Caution is needed in applying these data to the dietary management of neonates if energy intake is also reduced because data in parenterally fed neonates shows that a 30% reduction in total energy intake was associated with a 22% reduction in nitrogen retention (44). We are not aware of any comparable data in enterally fed neonates.
Abbreviations
- SSC:
-
Similac Special Care
REFERENCES
Pencharz PB, Parsons HG, Motil KJ, Duffy B 1981 Total body protein turnover and growth in children: is it a futile cycle?. Med Hypotheses 7: 155–160.
Raiha NCR 1980 Protein in the nutrition of the preterm infant: biochemical and nutritional considerations. In: Draper HH (ed) Advances in Nutritional Research. Plenum Press, New York, 173–206.
American Academy of Pediatrics, Committeeon Nutrition 1985 Nutritional needs of low-birth-weight infants. Pediatrics 75: 976–986
Canadian Paediatric Society Nutrition Committee 1995 Nutrient needs and feeding of premature infants. CMAJ 152: 1765–1785.
Pencharz PB, Steffee WP, Cochran W, Scrimshaw NS, Rand WM, Young VR 1977 Protein metabolism in human neonates: nitrogen-balance studies, estimated obligatory losses of nitrogen and whole body turnover of nitrogen. Clin Sci Mol Med 52: 485–495.
Young VR, Bier DM, Pellet PL 1989 A theoretical basis for increasing current estimates of the amino acid requirements in adult man, with experimental support. Am J Clin Nutr 45: 1323–1343.
Zello GA, Wykes LJ, Ball RO, Pencharz PB 1995 Recent advances in methods of assessing dietary amino acid requirements for adult humans. J Nutr 125: 2907–2915.
Zello GA, Pencharz PB, Ball RO 1990 Phenylalanine flux, oxidation and conversion to tyrosine in humans studied with L-[l-13C]phenylalanine. Am J Physiol 259:E835–E843.
Zello GA, Pencharz PB, Ball RO 1993 Dietary lysine requirement of young adult males determined by oxidation of L-[l-13C]phenylalanine. Am J Physiol 264:E677–E685.
Catzeflis C, Schutz Y, Micheli JL, Welsch C, Arnaud MJ, Jequier E 1985 Whole body protein synthesis and energy expenditure in very low birth weight infants. Pediatr Res 19: 679–687.
Duffy B, Pencharz P 1986 The effect of feeding route (IV or oral) on the protein metabolism of the neonate. Am J Clin Nutr 43: 108–111.
Pencharz PB, Beesley J, Sauer P, Van Aerde J, Canagarayar U, Renner J, McVey M, Wesson D, Swyer P 1989 A comparison of the estimates of whole-body protein turnover in parenterally fed neonates obtained using three different end products. Can J Physiol Pharmacol 67: 624–628.
Wutze KD, Heine W, Plath C, Müller M, Uhlemann M 1992 Whole-body protein parameters in premature infants: a comparison of different 15N tracer substances and different methods. Pediatr Res 31: 95–101.
Beaufrere B, Fournier V, Salle B, Putet G 1992 Leucine kinetics in fed low-birthweight infants: importance of splanchnic tissues. Am J Physiol 263:E214–E220.
Beaufrere B, Putet G, Pachiaudi C, Salle B 1990 Whole body protein turnover measured with 13C-leucine and energy expenditure in preterm infants. Pediatr Res 28: 147–152.
DeBenoist B, Abdulrazzak Y, Brooke OG, Halliday D, Millward DJ 1984 The measurement of whole body protein turnover in the pre-term infant with intragastric infusion of L-[l-13C]leucine and sampling of the urinary leucine pool. Clin Sci 66: 155–164.
Denne SC, Karn CA, Ahlrichs JA, Dorotheo AR, Wang J, Liechty EA 1996 Proteolysis and phenylalanine hydroxylation in response to parenteral nutrition in extremely premature and normal newborns. J Clin Invest 97: 746–754.
Denne SC, Karn CA, Liechty EA 1992 Leucine kinetics after a brief fast and in response to feeding in premature infants. Am J Clin Nutr 56: 899–904.
Denne SC, Karn CA, Liu YM, Lietch CA, Liechty EA 1994 Effect of enteral versus parenteral feeding on leucine kinetics and fuel utilization in premature newborns. Pediatr Res 36: 429–435.
Van Goudoever JB, Colen T, Wattimena JL, Huijmans JG, Carnielli VP, Sauer PJ 1995 Immediate commencement of amino acid supplementation in preterm infants: effect on serum amino acid concentrations and protein kinetics on the first day of life. J Pediatr 127: 458–465.
Van Goudoever JB, Sulkers EJ, Halliday D, Degenhart HJ, Carnielli VP, Wattimena JL, Sauer PJ 1995 Whole-body protein turnover in preterm appropriate for gestational age and small for gestational age infants: comparison of [15N]glycine and [1-13C]leucine administered simultaneously. Pediatr Res 37: 381–388.
van Toledo-Eppinga L, Houdijk MC, Delemarre-Van De Waal HA, Jakobs C, Lafeber HN 1996 Leucine and glucose kinetics during growth hormone treatment in intrauterine growth-retarded preterm infants. Am J Physiol 270:E451–E455.
Clark SE, Karn CA, Ahlrichs JA, Wang J, Leitch CA, Leichty EA, Denne SC 1997 Acute changes in leucine and phenylalanine kinetics produced by parenteral nutrition in premature infants. Pediatr Res 41: 568–574.
van Toledo-Eppinga L, Kalhan SC, Kulik W, Jakobs C, Lafeber HN 1996 Relative kinetics of phenylalanine and leucine in low birth weight infants during nutrient administration. Pediatr Res 40: 41–46.
Wykes LJ, Ball RO, Menendez CE, Ginther DM, Pencharz PB 1992 Glycine, leucine, and phenylalanine flux in low-birth-weight infants during parenteral and enteral feeding. Am J Clin Nutr 55: 971–975.
Zello GA, Telch J, Clarke R, Ball RO, Pencharz PB 1992 Reexamination of protein requirements in adult males by end-product measurements of leucine and lysine kinetics. J Nutr 122: 1000–1008.
Bross R, Ball RO, Clarke JTR, Pencharz PB 2000 Tyrosine requirements in children with classical PKU determined by indicator amino acid oxidation. Am J Physiol 278:E195–E201.
Dubowitz LM, Dubowitz V, Goldberg C 1970 Clinical assessment of gestational age in the newborn infant. J Pediatr 77: 1–10.
Darling PB, Dunn M, Sarwar G, Brookes S, Ball RO, Pencharz PB 1999 Threonine kinetics in preterm infants fed their mothers' milk or formula with various ratios of whey to casein. Am J Clin Nutr 69: 105–114.
Jones PJH, Pencharz PB, Clandinin MT 1985 Whole body oxidation of dietary fatty acids: implications for energy utilization. Am J Clin Nutr 42: 769–777.
Ford GC, Cheng KN, Halliday D 1985 Analysis of [1-13C]leucine and [13C]KIC in plasma by capillary gas chromatographymass spectrometry in protein turnover studies. Biomed Mass Spectrom 12: 432–436.
Matthews DE, Motil KJ, Rohrbaugh DK, Burke JF, Young VR, Bier DM 1980 Measurement of leucine metabolism in man from a primed, continuous infusion of L-[l-13C]leucine. Am J Physiol 238:E473–E479.
Van Aerde JEE, Sauer PJJ, Pencharz PB, Canagarayar U, Beesley J, Smith JM, Swyer PR 1985 The effect of energy intake and expenditure on the recovery of 13CO2 in the parenterally fed neonate during a 4-hour primed constant infusion of NaH13CO3. Pediatr Res 19: 806–810.
Picou D, Taylor-Roberts T 1969 The measurement of total protein synthesis and catabolism and nitrogen turnover in infants in different nutritional states and receiving different amounts of dietary protein. Clin Sci 36: 283–296.
Munro MH, Fleck A Analysis of tissue and body fluids for nitrogenous constituents. In: Munro HN (ed) Mammalian Protein Metabolism. Academic Press, New York, pp 424–425
Pencharz PB, Farri L, Pagageorgiou A 1983 The effects of human milk and lowprotein formulae on the rates of total body protein turnover and urinary 3-methylhistidine excretion of preterm infants. Clin Sci 64: 611–616.
Snedecor GW, Cochran WG 1989 Statistical Methods, 8th ed. Iowa State University Press, Ames, IA
SAS Institute 1991 SAS/STAT Guide for Personal Computers. SAS Institute, Inc., Cary, NC
Synderman SE, Boyer A, Kogut MD, Holt LE 1963 The protein requirement of the premature infant. I. The effect of protein intake on the retention of nitrogen. J Pediatr 74: 872–880.
Roberts SA, Ball RO, Moore AM, Filler RM, Pencharz, PB 2001 The effect of graded intake of glycyl-tyrosine on phenylalanine and tyrosine metabolism in parenterally fed neonates with an estimate of tyrosine requirements. Pediatr Res 49: 111–119.
Bertolo RFP, Chen ZL, Law G, Pencharz PB, Ball RO 1998 Threonine requirement of neonatal piglets receiving total parenteral nutrition is considerable lower than that of piglets receiving an identical diet intragastrically. J Nutr 128: 1752–1759.
Ball RO, Bayley HS 1986 Influence of dietary protein concentration on the oxidation of phenylalanine by the young pig. Br J Nutr 55: 651–658.
Rand WM, Scrimshaw NS, Young VR 1976 Change of urinary nitrogen excretion in response to low-protein diets in adults. Am J Clin Nutr 29: 639–644.
Duffy B, Gunn T, Collinge J, Pencharz PB 1981 The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (<1600 g) infants. Pediatr Res 15: 1040–1044.
Acknowledgements
We thank Dr. L. Marai for mass spectrometry analysis and D. Malyon Ginther for nursing assistance. We also acknowledge the efforts of the staff in the neonatal intensive care unit and pharmacy at The Hospital for Sick Children. To the families of the babies, we greatly appreciate your cooperation and interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by the Canadian Institutes of Health (Grant #12928). Experimental formulae were supplied by Wyeth-Ayerst Laboratories, Philadelphia, PA.
Rights and permissions
About this article
Cite this article
Zello, G., Menendez, C., Rafii, M. et al. Minimum Protein Intake for the Preterm Neonate Determined by Protein and Amino Acid Kinetics. Pediatr Res 53, 338–344 (2003). https://doi.org/10.1203/01.PDR.0000046760.25817.0B
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/01.PDR.0000046760.25817.0B