Abstract
This prospective randomized pilot study aimed to test the hypotheses that partial liquid ventilation combined with a high positive end-expiratory pressure (PEEP) and a moderate tidal volume results in improved gas exchange and lung mechanics without negative hemodynamic influences compared with conventional mechanical ventilation in acute lung injury in piglets. Acute lung injury was induced in 12 piglets weighing 9.0 ± 2.4 kg by repeated i.v. injections of oleic acid and repeated lung lavages. Thereafter, the animals were randomly assigned either to partial liquid ventilation (n = 6) or conventional mechanical ventilation (n = 6) at a fractional concentration of inspired O2 of 1.0, a PEEP of 1.2 kPa, a tidal volume < 10 mL/kg body weight (bw), a respiratory rate of 24 breaths/min, and an inspiratory/expiratory ratio of 1:2. Perfluorocarbon liquid 30 mL/kg bw was instilled into the endotracheal tube over 10 min followed by 5 mL/kg bw/h. Continuous monitoring included ECG, mean right atrial, pulmonary artery, pulmonary capillary, and arterial pressures, arterial blood gas, and partial pressure of end-tidal CO2 measurements. When compared with control animals, partial liquid ventilation resulted in significantly better oxygenation with improved cardiac output and oxygen delivery. Dead space ventilation appeared to be lower during partial liquid ventilation compared with conventional mechanical ventilation. No significant differences were observed in airway pressures, pulmonary compliance, and airway resistance between both groups. The results of this pilot study suggest that partial liquid ventilation combined with high PEEP and moderate tidal volume improves oxygenation, dead space ventilation, cardiac output, and oxygen delivery compared with conventional mechanical ventilation in acute lung injury in piglets but has no significant influence on lung mechanics.
Similar content being viewed by others
Main
ARDS is characterized by acute inflammatory lung injury finally resulting in end-expiratory alveolar collapse, atelectasis, increase in right-to-left shunt, and a decrease in oxygenation (1). The goals of mechanical ventilation should be to maintain lung function and to preserve lung architecture. PEEP is usually applied to prevent end-expiratory collapse of collapsible alveoli, and tidal volume is selected to limit overdistension of lung units (2, 3). However, certain lung regions, mainly in the dependent parts of the lung, are often poorly responsive to PEEP, whereas other regions in the nondependent parts of the lung are very responsive to PEEP (4). Another therapeutic option would be to eliminate the increased forces acting at the air-liquid interface in ARDS by filling the lungs with a perfluorochemical liquid (5).
In recent years, liquid ventilation with perfluorocarbon has gained increasing interest for improving pulmonary gas exchange in experimental models of acute lung injury (6–8). Perfluorocarbons are hydrocarbon molecules in which carbon-bound hydrogen is replaced by fluorine atoms. They are biologically inert, nonbiotransformable. and immiscible in both aqueous and lipid media. Perfluorocarbon liquids are absorbed minimally by the respiratory epithelium and are eliminated by evaporation through the lungs (9). Oxygenated perfluorocarbon liquids have the ability to lower surface tension in surfactant-deficient lungs, to increase functional residual capacity, and are able to dissolve large amounts of respiratory gases. Fuhrman et al.(10) simplified the application of liquid ventilation by combining liquid and gas ventilation. The lungs are filled with perfluorocarbon to their estimated functional residual capacity (30 mL/kg bw), or liquid is added until at end expiration at 0 PEEP a meniscus is seen in the endotracheal tube and subsequently ventilated by a conventional gas ventilator using the volume-controlled mode with PEEP levels < 0.6 kPa and a tidal volume of 15 mL/kg bw (8, 10). Because of its high density, perfluorocarbon is mainly distributed to dependent lung regions (11). As a noncompressible liquid, it preferentially stabilizes collapsed alveoli in the dependent parts of the lung (“liquid PEEP”). Gas exchange during partial liquid ventilation most likely occurs at the alveolar level both in aerated and liquid filled lung units.
Partial liquid ventilation has been shown to improve lung mechanics, oxygenation, and to decrease further alveolar damage (7, 8, 12–14). However, little is known about the optimal gas ventilatory strategy during partial liquid ventilation. Recently, Cox et al.(15) reported a significant risk of barotrauma during partial liquid ventilation when lungs are filled with perfluorocarbon liquid to functional residual capacity levels with a superimposed gas ventilatory strategy of large tidal volumes. Most of the controlled experimental studies on partial liquid ventilation used volume controlled mechanical ventilation with large tidal volumes and low PEEP levels in the partial liquid ventilation as well as in the control groups (6–8, 12, 16). However, there is some evidence that conventional mechanical ventilation with PEEP levels above the lower inflection point combined with low tidal volumes reduces lung injury in experimental studies of acute lung injury (17–19).
In this pilot study, we compared the effects of partial liquid ventilation and conventional mechanical ventilation on gas exchange, respiratory mechanics, and hemodynamics in an animal model of acute lung injury using identical gas ventilatory settings with high PEEP and moderate tidal volume in both groups.
We hypothesized that partial liquid ventilation results in improved gas exchange and lung mechanics compared with conventional mechanical ventilation without negative hemodynamic influences.
MATERIALS AND METHODS
Animals.
The protocol was approved by the institutional animal research committee, and the care of the animals was in accordance with guidelines for ethical animal research.
In 12 piglets of either sex, weighing 9.0 ± 2.4 kg, premedicated with azaperone (8 mg/kg bw i.m.) and atropine (0.02 mg/kg bw i.m.), anesthesia was induced with ketamine (10 mg/kg bw i.v.) and thereafter maintained by a continuous infusion of fentanyl (0.15 μg/kg bw/min), pentobarbital sodium (4 mg/kg bw/h), and pancuronium (0.3 mg/kg bw/h). All animals were placed in supine position. After placing a 5.5 mm (inner diameter) tracheal Hi-Lo cuffed tube (Hi-Lo jet tube, National Catheter Corp., Mallinckrodt, Glen Falls, NY, USA) via tracheostomy, controlled mechanical ventilation was established using the adaptive pressure ventilation option of the Galileo ventilator (Hamilton Medical AG, Rhäzüns, Switzerland) with a 15 mm pediatric breathing system (Intersurgical Inc., Berkshire, UK). Tidal volume was set at 10 to 12 mL/kg bw, respiratory rate at 24 breath/min, inspiratory/expiratory ratio at 1:2, PEEP at 0.4 kPa, and fraction of inspired oxygen (FiO2) at 0.3. Initially, a 5 Fr triple-lumen catheter (Hydrocath 5 Fr 15 cm 3 lumen, Ohmeda, Swindon, UK) was inserted into the right subclavian vein to induce anesthesia and to administer maintenance fluid. After induction of anesthesia, a ringer solution was infused, initially, at a rate of 20 mL/kg bw in 30 min followed by a continuous infusion of 5 mL/kg bw/h. A 5.5 Fr thermodilution oxygen-saturation fiber-optic pulmonary artery catheter (Edwards Swan-Ganz TD Catheter, Edwards Critical Care Division, Irvine, CA, USA) was placed into the pulmonary artery by peripheral cutdown of the right external jugular vein. A 4 Fr O2-saturation catheter (Edslab double lumen O2 Sat II catheter, Edwards Critical Care Division) was inserted via the right common carotid artery and placed into the thoracic aorta for continuous arterial oximetry measurement. A short 18 gauge catheter (Abbocath, Abbott Ireland LTD, Sligo, Republic of Ireland) was inserted via the left common carotid artery through which a thin optical sensor (Multiparameter Intravascular Sensor MPS7004S <0.5 mm diameter, Biomedical Sensors Ltd, Malvern, PA, USA) was advanced into the aortic arch for continuous blood gas and arterial pressure monitoring using the TrendCare TCM 7000 system (Diametrics Medical Ltd, Buckinghamshire, England). A second 5 Fr triple-lumen catheter (Hydrocath 5 Fr 15 cm 3 lumen, Ohmeda Swindon, UK) was placed via the left external jugular vein into the superior vena cava.
Hemodynamic monitoring.
ECG, mean right atrial, pulmonary artery, pulmonary capillary, and arterial pressures were recorded continuously on a multichannel recorder (SC 8000, Siemens Medical Systems, Inc., Danvers, MA, USA) using 0.9% saline filled transducers (Monitoring Kit Transpac IV, H674/61, Abbott Critical Care Systems, Sligo, Republic Of Ireland). All pressures were referred to a zero level taken at the middle of the ventral to dorsal thoracic distance. Cardiac output was measured at end expiration by thermodilution technique as the mean of three determinations after injection of 5 mL 0°C 0.9% saline. Systemic and pulmonary vascular resistances were calculated using the following equations: systemic vascular resistance, [(arterial pressure − right atrial pressure) * 60]/cardiac output; pulmonary vascular resistance, [(pulmonary artery pressure − pulmonary capillary pressure) * 60]/cardiac output).
Respiratory monitoring.
Arterial and mixed venous blood samples were taken for measurements of blood gases and Hb concentration using an automatic blood gas system (AVL 995, AVL Corp., Graz, Austria). In addition, the arterial and mixed venous oxygen saturations (CO-Oxylite AVL 912, AVL) were analyzed. End-tidal CO2 concentrations were measured with an end-tidal CO2 module of the Siemens SC 8000 monitor using the mainstream technique. Barometric pressure was noted daily during the experiment. The alveolar-arterial oxygen tension difference (PA − aO2) was calculated using the following formula: PA − aO2 = [FiO2 * (Patm − PH2O) − (PaCO2/0.8) − PaO2], where FiO2 is fraction of inspired oxygen, Patm is barometric pressure, PH2O is partial pressure of H2O, PaCO2 is partial pressure of arterial CO2, and PaO2 is partial pressure of arterial O2. During partial liquid ventilation, the partial pressure of the perfluorocarbon fluid at 37°C was included in the calculation of PA − aO2 = [FiO2 * (Patm − PH2O − Pperfluorocarbon) − (PaCO2/0.8) − PaO2], where Pperfluorocarbon is partial pressure of perfluorocarbon. Intrapulmonary shunt fraction was calculated using the following formula: [Q′s/Q′t = (Cc′O2 −CaO2)/(Cc′O2 − CvO2)], where Cc′O2 is ideal pulmonary capillary oxygen content, CaO2 is arterial oxygen content, and CvO2 is mixed venous oxygen content. Dead space-to-tidal volume ratio (Vd/Vt is physiologic dead space) was calculated according to a modified Bohr alveolar equation: Vd/Vt = [(PaCO2 − PetCO2)/PaCO2], where, PaCO2 is partial pressure of arterial CO2, and PetCO2 is partial pressure of end-tidal CO2. For this measurement only, stable capnography curves with a zero baseline and an expiratory plateau phase over a period of 10 ventilatory cycles were taken.
Respiratory mechanics, inspiratory and expiratory gas flow rates, and airway pressures were monitored at the proximal airway using the Hamilton flow sensor (Hamilton Medical AG, Rhäzüns, Switzerland), which was calibrated at the beginning of mechanical ventilation in all animals. Plateau pressure and inspiratory tidal volume were measured after a 5-s inspiratory breath hold followed by waveform freezing and cursor measurement. Total PEEP was measured with an end-expiratory pressure hold of 5 s. These measurements were only done after 10 stable pressure, volume, and flow curves were observed on the monitoring screen of the ventilator. Static pulmonary compliance was calculated from the inspiratory tidal volume and the static inspiratory and expiratory pressures according to the equation Cstat: Tidal volume/(Pplateau − PEEPtot). Measurements of tidal volume and pulmonary compliance were indexed to body weight in kilograms. Inspiratory and expiratory resistances were calculated by the Galileo ventilator using the least squares fit method (20).
Protocol.
After instrumentation, the animals were allowed to rest for 30 min before baseline measurements were performed. Before induction of acute lung injury, the FiO2 of the ventilator was increased to 1.0 and the PEEP level to 0.6 kPa. Acute respiratory failure was initially induced by repeated injections of oleic acid (0.01 mL/kg bw) into the right atrium followed by repeated lung lavages using warmed 0.9% saline (20 mL/kg bw/lavage). The lung lavage was repeated every 10 min until the PaO2/FiO2 ratio fell below 13 kPa. During the induction of acute lung injury, inotropic support with dopamine 10 μg/kg bw/min was instituted. After induction of acute lung injury, the animals were allowed to stabilize for at least 30 min. Thereafter, the animals were randomly assigned to either partial liquid ventilation (n = 6) or control (n = 6) groups using sealed envelopes. Both groups were ventilated with adaptive pressure ventilation using a tidal volume of < 10 mL/kg bw, a PEEP of 1.2 kPa, an inspiratory/expiratory ratio of 1:2, a respiratory rate of 24 breaths/min, and a FiO2 of 1.0.
Partial liquid ventilation was started using the Rimar 101 perfluorocarbon liquid (Miteni, Milan, Italy). Rimar 101 has a specific gravity of 1.77 g/mL at 25°C, a surface tension of 1.47 dyne/kPa, a vapor pressor of 8.5 kPa at 37°C, an oxygen solubility of 52 mL/100 mL, a CO2 solubility of 160 mL/100 mL at 37°C, and 1 atm of pressure. The perfluorocarbon liquid was oxygenated at an FiO2 of 1.0 and warmed to 38°C. Thereafter, in the partial liquid ventilation group, the oxygenated perfluorocarbon liquid was instilled into the lung up to 30 mL/kg bw or a visible meniscus at the endotracheal tube at end expiration without PEEP. Measurements of all cardiopulmonary variables were repeated every 30 min up to 120 min. Evaporative losses of the liquid were compensated by adding 5 mL/kg bw/h of the oxygenated perfluorocarbon liquid without any further control of a visible meniscus at the endotracheal tube at 0 PEEP. To avoid severe acidosis (pH < 7.20), tris was infused at a rate of 0.01 mmol/kg bw/min in both groups.
After the end of the experiment, the animals were killed with an overdose of sodium pentobarbital and a rapid injection of potassium chloride.
Data analysis.
All statistical analyses on recorded data were performed using the Statview 4.5 software for Macintosh. Descriptive statistics were performed on all cardiorespiratory variables and are depicted as median and interquartile range. A comparison of baseline and postinjury data between groups was made by unpaired t test. As the assumptions on which parametric tests are based were not always fulfilled during the study period, a summary measure was derived for each group including all data from postlung injury up to 2 h of mechanical ventilation (21). The area under the curve (AUC) is the product of the time difference and average of two consecutive measurements [AUC1: (t2 − t1) * (y1 + y2)/2] and is given in U/h. It was calculated using the MS Excel software 98 for Macintosh. The total AUC was calculated as the sum of all half-an-hourly based areas between acute lung injury and 2 h (AUCtot: AUC1 + AUC2 + AUC3 + AUC4). Thereafter, the summary data were compared using the nonparametric Mann Whitney U-test. A p value of <0.05 was considered significant.
RESULTS
Twelve piglets, six in each group, were studied. Age, body weight, medication, and instrumentation during the experiment were similar for animals in both groups. Baseline cardiorespiratory variables were similar in both groups (Tables 1 and 2). Seven to nine repeated injections of oleic acid (0.01 mL/kg bw) combined with five to eight repeated lung lavages (20 mL/kg bw) in both groups induced a stable model of acute lung injury with no significant differences in cardiorespiratory variables between both groups (Tables 1 and 2).
Gas exchange.
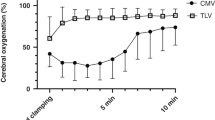
Blood gas, acid base, and respiratory variables are summarized in Table 1. As shown in Figure 1, PaO2/FiO2 values were significantly higher during partial liquid ventilation than during conventional mechanical ventilation. Simultaneously, intrapulmonary shunt fraction and alveolar-arterial oxygen tension difference were significantly lower during partial liquid ventilation than during conventional mechanical ventilation, whereas overall PaCO2 and pH values were not significantly different between both groups.
Box plot graph of PaO2/FiO2 during partial liquid ventilation and conventional mechanical ventilation in an animal model of acute lung injury. The box plot displays 10th, 25th, 50th, 75th, and 90th percentiles of PaO2/FiO2. First summary data were derived for each group, including values postlung injury. Up to 2 hr of mechanical ventilation and then the summary data between both groups were compared using the nonparametric Mann-Whitney U-test. ALI, acute lung injury; BL, baseline; PLV, partial liquid ventilation.
Respiratory mechanics.
As shown in Table 1, there were no significant differences in airway pressures, static pulmonary compliance, and inspiratory and expiratory airway resistances between both groups. Dead space ventilation appeared to be lower in the partial liquid ventilation group when compared with the control group.
Hemodynamics.
Hemodynamic data are presented in Table 2. Whereas there were no significant differences in HR, right atrial, pulmonary artery, and systemic arterial pressures between both groups, cardiac output and oxygen delivery were significantly higher during partial liquid ventilation when compared with control animals. The higher cardiac output combined with unchanged pulmonary artery pressure resulted in a significantly lower pulmonary vascular resistance in the partial liquid ventilation group compared with the control group.
All animals survived the 2-h trial.
DISCUSSION
The main findings of this pilot study suggest that (1) oxygenation was significantly better in the partial liquid ventilation group when compared with the control group and (2) cardiac output and oxygen delivery were significantly higher during partial liquid ventilation compared with conventional mechanical ventilation. An unexpected finding was that dead space appeared to be lower during partial liquid ventilation than during conventional mechanical ventilation.
The rationale for mechanical ventilation in acute lung injury is to apply a PEEP to prevent end-expiratory collapse of collapsible alveoli and to select a tidal volume to limit overdistension of lung units (22). Another therapeutic option would be to eliminate the increased forces acting at the air–liquid interface in the acutely damaged lung by filling the lungs with a perfluorochemical liquid.
Partial liquid ventilation.
In 1991, Fuhrman et al.(10) introduced the technique of perfluorocarbon-associated gas exchange or partial liquid ventilation. This new technique combined liquid ventilation at functional residual capacity and tidal gas volume ventilation using a conventional ventilator. Meanwhile, a number of experimental studies have shown that partial liquid ventilation significantly improves oxygenation, lung mechanics, decreases further alveolar damage, and decreases serum tumor necrosis factor-α concentration in acute lung injury (6–8, 12, 14, 16, 23–25). Most investigators filled the lung with perfluorocarbon liquid at functional residual capacity level (30 mL/kg bw) and used volume-controlled mechanical ventilation with a tidal volume of 15 mL/kg bw and a PEEP level between 0.2 and 0.6 kPa in both the partial liquid ventilation as well as the control groups.
Recently, Cox et al.(15) reported a significant risk of barotrauma during partial liquid ventilation when lungs are filled with perfluorocarbon liquid to functional residual capacity levels with a superimposed gas ventilatory strategy of large tidal volumes. In 1999, we reported that the application of PEEP 1.0 to 1.2 kPa during partial liquid ventilation seems to be optimal to improve oxygenation and lung mechanics without any negative hemodynamic side effects (26). Recently, Kirmse et al.(27) set PEEP levels according to the lower inflection point + 0.1 kPa in a lung lavage model of acute lung injury resulting in PEEP values between 1.1 and 1.3 kPa associated with improved oxygenation and lung mechanics. In addition, the same group reported that with PEEP set at the lower inflection point, adequate gas exchange and improved lung mechanics can be obtained in both volume and pressure controlled modes of mechanical ventilation (28). According to these data, we now used adaptive pressure ventilation with a PEEP of 1.2 kPa, a tidal volume of <10 mL/kg bw, and a fixed ventilator rate of 24 breaths/min. A tidal volume of <10 mL/kg bw was selected to reduce the risk of barotrauma, and the ventilator rate was not increased to compensate for moderate hypercapnia. The advantage of adaptive ventilation is that the set target volume is achieved with the lowest pressure possible depending on lung mechanics.
Oxygenation.
In healthy lungs, partial liquid ventilation increases O2 shunt and arterial-alveolar CO2 difference due to diffusion limitation, ventilation/perfusion heterogeneity, and intrapulmonary shunt (14, 24, 29). However, oxygenation is significantly improved during partial liquid ventilation in a variety of models of acute lung injury due to recruitment of collapsed lung regions, decrease in surface tension, and redistribution of blood flow to nondependent lung regions (6–8, 12, 29). In our pilot study, this improved oxygenation during partial liquid ventilation is confirmed using a high PEEP and moderate tidal volume gas ventilatory strategy. We speculate that partial liquid ventilation combined with a high PEEP ventilatory strategy might stabilize dependent as well as nondependent lung units, resulting in improved ventilation/perfusion heterogeneity and oxygenation.
Ventilation.
In this pilot study, both ventilatory strategies with tidal volumes < 10 mL/kg bw resulted in moderate hypercapnia with no significant difference between both groups. In contrast, literature reports improved ventilation during partial liquid ventilation using tidal volumes > 12.5 mL/kg bw (7, 12, 30). This difference in ventilation might be caused by the low gas volume ventilatory strategy and the CO2 solubility and diffusibility of the perfluorocarbon fluid used. Whereas there are very few data on dead space ventilation during partial liquid ventilation, Murray et al.(31) already reported in 1984 that an arterial-end tidal CO2 pressure gradient would be smallest when there is maximal alveolar recruitment by PEEP without alveolar overdistension in acute lung injury. The literature reports both an increase and no change in dead space ventilation during partial liquid ventilation in healthy as well as in acutely injured lungs (29, 32–34). Mates et al.(32) speculated that a diffusion barrier imposed by a layer of perfluorocarbon might be responsible for this increased dead space ventilation in healthy piglets. In contrast to these findings, in our pilot study, dead space ventilation appeared to decrease rapidly during partial liquid ventilation, whereas in the control group with similar gas ventilatory parameters, dead space appeared to be significantly higher with some decrease over time. We speculate that the use of higher PEEP levels during partial liquid ventilation would improve perfluorocarbon distribution within the lung, would redistribute pulmonary blood flow to nondependent parts of the lung, and would improve ventilation/perfusion heterogeneity, resulting in lower physiologic dead space. However, there are some limitations to calculate dead space during partial liquid ventilation using the Bohr equation 1. The basic assumption of the Bohr equation to calculate physiologic dead space is that pulmonary capillary blood fully equilibrates with alveolar gas (35). However, this complete arterial-alveolar equilibration is probably not given during partial liquid ventilation because of diffusion limitation and heterogeneity of perfluorocarbon distribution (36). Therefore, Mates et al.(37) proposed to use the multiple inert gas elimination technique to measure physiologic dead space during partial liquid ventilation and observed no significant difference between partial liquid ventilation and gas ventilation in healthy piglets. For the classical Bohr equation, the mean partial pressure of CO2 in the expired gas and not the end-tidal CO2 is used. High levels of PEEP may significantly increase the slope of the alveolar plateau, resulting in falsely low dead space values (38). In our pilot study, expiratory CO2 curves were very stable without any positive or negative slope of the alveolar plateau both during partial liquid ventilation and conventional mechanical ventilation. On the basis of our stable expiratory CO2 curves, we might exclude dead space calculation errors either induced by high PEEP levels or by the heterogeneity of perfluorocarbon distribution in the lung. Our contradictory results on ventilation and physiologic dead space during partial liquid ventilation might be explained on the one hand by diffusion limitation combined with improved ventilation/perfusion heterogeneity and on the other hand by the limitations of the modified Bohr equation during partial liquid ventilation.
Respiratory mechanics.
There are several reports on improved static compliance of the respiratory system in different models of acute lung injury during partial liquid ventilation (8, 12, 27, 30, 39, 40). However, compliance measurements might be problematic during partial liquid ventilation because the gas-volume based compliance measurements are restricted to the gas filled parts of the lung and may not reflect the whole lung compliance. Recently, Hirschl et al.(12) demonstrated that compliance measurements are significantly different in the same animal when gas versus liquid is used to assess compliance. In 1998, Kirmse et al.(27) noticed a significantly higher static compliance of the respiratory system at high PEEP levels compared with low PEEP levels in sheep with a lung lavage induced model of acute lung injury. These findings were confirmed by our study in piglets with acute lung injury induced by oleic acid infusion and repeated lung lavages when PEEP levels were increased from 0 to 1.5 kPa during partial liquid ventilation (26). In this pilot study, our hypothesis that pulmonary mechanics would be significantly improved during partial liquid ventilation compared with conventional mechanical ventilation was not confirmed. In contrast to the previous study, we now used much lower tidal volumes, which might have influenced pulmonary compliance in this model of acute lung injury (26). In addition, differences in compliance data during partial liquid ventilation might be explained by different measuring techniques (quasi-static versus static technique with or without PEEP) and by the differences in lung injury models in different animals. In this pilot study, we used inspiratory and expiratory hold techniques of 5 s and the inspiratory tidal volume for compliance measurement on the one hand to obtain reliable static pressure conditions and on the other hand to avoid influences of the high vapor pressure of the perfluorocarbon liquid on expiratory flow and volume measurements.
The literature reports on both increases and decreases in airway resistance during partial liquid ventilation depending on the lung injury model and the gas ventilatory strategy superimposed to partial liquid ventilation (6, 7, 40). In this pilot study, no differences in inspiratory and expiratory airway resistances were noticed between partial liquid ventilation and conventional mechanical ventilation using similar gas ventilatory parameters.
Hemodynamics.
During total liquid ventilation, hemodynamic compromise was reported, which was not observed later during partial liquid ventilation even in large animals (41–43). Hemodynamic compromise was speculated to result from pulmonary vascular compression due to the high hydrostatic pressure induced by the high specific gravity of the perfluorocarbon fluid. In 1995, Houmes et al.(44) reported that partial liquid ventilation significantly improves gas exchange and does not lead to hemodynamic depression in large pigs with lung lavage induced acute lung injury. In addition, these authors observed an improvement in pH during partial liquid ventilation and speculated that a stable cardiac output and oxygen delivery would be responsible for this improvement. In our pilot study, cardiac output and oxygen delivery were significantly improved during partial liquid ventilation compared with conventional mechanical ventilation. One reason might be that cardiac output was slightly higher at baseline and after inducing acute lung injury in the partial liquid ventilation group than in the control group. In addition, we speculate that improved oxygenation and higher PaCO2 values over time might have influenced cardiac output in the partial liquid ventilation treated animals.
Limitations of the study.
Because of the small sample size in each group (n = 6), a large beta error may have existed, that is, some of the comparisons that did not reach significance might have done so with a larger number in each group. Another limitation of this pilot study is the short duration of the experiment. Clinically, it would be very important to evaluate cardiorespiratory data for a longer period of time combined with radiologic and histologic evaluation of the lungs. Although we used a “lung protective” gas ventilatory strategy in both groups, lung protection was not the focus of this pilot study, and further research is needed before protective effects of PEEP during partial liquid ventilation can be established.
CONCLUSION
The results of this pilot study suggest that partial liquid ventilation combined with a superimposed gas ventilatory strategy including high PEEP and moderate tidal volume significantly improves oxygenation, cardiac output, and oxygen delivery compared with conventional mechanical ventilation in acute lung injury in piglets but has no significant influence on lung mechanics. In addition, dead space ventilation appeared to be lower during partial liquid ventilation compared with conventional mechanical ventilation. However, further studies are required to assess the optimal gas ventilatory strategy during partial liquid ventilation to obtain adequate gas exchange, stable hemodynamics, and lung protection.
Abbreviations
- ARDS:
-
Acute respiratory distress syndrome
- HR:
-
Heart rate
- PEEP:
-
Positive end-expiratory pressure
References
Royall JA, Levin DL 1988 Adult respiratory distress syndrome in pediatric patients. I. Clinical aspects, pathophysiology, pathology mechanisms of lung injury. J Pediatr 112: 169–180
Lachmann B 1992 Open up the lung keep the lung open. Intensive Care Med 18: 319–321
The Acute Respiratory Distress Syndrome Network 2000 Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury the acute respiratory distress syndrome. N Engl J Med 342: 1301–1308
Gattinoni L, D'Andrea L, Pelosi P, Vitale G, Pesenti A, Fumagalli R 1993 Regional effects mechanism of positive end-expiratory pressure in early adult respiratory distress syndrome. JAMA 269: 2122–2127
Lachmann B, Verbrugge S 1996 Liquid ventilation. Curr Opin Crit Care 2: 60–66
Tütüncü AS, Faithfull NS, Lachmann BL 1993 Intratracheal perfluorocarbon administration combined with mechanical ventilation in experimental respiratory distress syndrome: dose-dependent improvement of gas exchange. Crit Care Med 21: 962–969
Leach CL, Fuhrman BP, Morin FC, Rath MG 1993 Perfluorocarbon-associated gas exchange (partial liquid ventilation) in respiratory distress syndrome: a prospective, randomized, controlled study. Crit Care Med 21: 1270–1278
Papo MC, Paczan PR, Fuhrman BP, Steinhorn DM, Hernan LJ, Leach CL, Holm BA, Fisher JE, Kahn BA 1996 Perfluorocarbon-associated gas exchange improves oxygenation, lung mechanics, survival in a model of adult respiratory distress syndrome. Crit Care Med 24: 466–474
Wolfson MR, Greenspan JS, Shaffer TH 1998 Liquid-assisted ventilation: an alternative respiratory modality. Pediatr Pulmonol 26: 42–63
Fuhrman BP, Paczan PR, DeFrancisis M 1991 Perfluorocarbon-associated gas exchange. Crit Care Med 19: 712–722
Quintel M, Hirschl RB, Roth H, Loose R, Tillmanns R, van Ackern K 1998 Computer tomographic assessment of perfluorocarbon distribution gas distribution during partial liquid ventilation for acute respiratory failure. Am J Respir Crit Care Med 158: 249–255
Hirschl RB, Tooley R, Parent AC, Johnson K, Bartlett RH 1996 Evaluation of gas exchange, pulmonary compliance, lung injury during total partial liquid ventilation in the acute respiratory distress syndrome. Crit Care Med 24: 1001–1008
Quintel M, Heine M, Hirschl RB, Tillmanns R, Wessendorf V 1998 Effects of partial liquid ventilation on lung injury in a model of acute respiratory failure: a histologic morphometric analysis. Crit Care Med 26: 833–843
Rotta AT, Gunnarsson B, Hernan LJ, Fuhrman BP, Steinhorn DM 1999 Partial liquid ventilation influences pulmonary histopathology in an animal model of acute lung injury. J Crit Care 14: 84–92
Cox PN, Frndova H, Tan PSK, Nakamura T, Miyasaka K, Sakurai Y, Middleton W, Mazer D, Bryan AC 1997 Concealed air leak associated with large tidal volumes in partial liquid ventilation. Am J Respir Crit Care Med 156: 992–997
Overbeck MC, Pranikoff T, Yadao CM, Hirschl RB 1996 Efficacy of perfluorocarbon partial liquid ventilation in a large animal model of acute respiratory failure. Crit Care Med 24: 1208–1214
Sandhar BK, Niblett DJ, Argiras EP, Dunnill MS, Syskes MK 1988 Effects of positive end-expiratory pressure on hyaline membrane formation in a rabbit model of the neonatal respiratory distress syndrome. Intensive Care Med 14: 538–546
Muscedere JG, Mullen JBM, Gan K, Slutsky AS 1994 Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149: 1327–1334
Corbridge TC, Wood LDH, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI 1990 Adverse effects of large tidal volume low PEEP in canine acid aspiration. Am J Respir Crit Care Med 142: 311–315
Iotti GA, Braschi A, Brunner JX, Smits T, Olivei M, Palo A, Veronesi R 1995 Respiratory mechanics by least squares fitting in mechanically ventilated patients: applications during paralysis during pressure support ventilation. Intensive Care Med 21: 406–413
Matthews JNS, Altman DG, Campbell MJ, Royston P 1990 Analysis of serial measurements in medical research. BMJ 300: 230–235
Amato MBP, Barbas CSV, Medeiros DM, Magaldi RB, Schettino GDPP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CRR 1998 Effect of a protective ventilation strategy on the mortality in the acute respiratory distress syndrome. N Engl J Med 338: 347–354
Zobel G, Urlesberger B, Dacar D, Rödl S, Reiterer F, Friehs I 1997 Partial liquid ventilation combined with inhaled nitric oxide in acute respiratory failure with pulmonary hypertension in piglets. Pediatr Res 41: 172–177
Hernan LJ, Fuhrman BP, Kaiser RE, Penfil S, Foley C, Papo MC, Leach CL 1996 Perfluorocarbon-associated gas exchange in normal acid-injured large sheep. Crit Care Med 24: 475–481
Kawamae K, Pristine G, Chiumello D, Tremblay LN, Slutsky AS 2000 Partial liquid ventilation decreases serum tumor necrosis factor-a concentrations in a rat acid aspiration lung injury model. Crit Care Med 28: 479–483
Zobel G, Rödl S, Urlesberger B, Dacar D, Trafojer U, Trantina A 1999 The effect of positive end-expiratory pressure during partial liquid ventilation in acute lung injury in piglets. Crit Care Med 27: 1934–1939
Kirmse M, Fujino Y, Hess D, Kacmarek M 1998 Positive end-expiratory pressure improves gas exchange pulmonary mechanics during partial liquid ventilation. Am J Respir Crit Care Med 158: 1550–1556
Fujino Y, Kirmse M, Hess D, Kacmarek M 1999 The effect of mode, inspiratory time, positive end-expiratory on partial liquid ventilation. Am J Respir Crit Care Med 159: 1087–1095
Enrione MA, Papo MC, Leach CL, Holm BA, Hernan LJ, Fuhrman BP, Dowhy MS, Rath MG, Frisicaro PE 1999 Regional pulmonary blood flow during partial liquid ventilation in normal acute oleic acid-induced lung-injured piglets. Crit Care Med 27: 2716–2723
Wilcox DT, Glick PL, Karamanoukian HL, Leach C, Morin FC, Fuhrman BP 1995 Perfluorocarbon-associated gas exchange improves pulmonary mechanics, oxygenation, ventilation, allows nitric oxide delivery in the hypoplastic lung congenital diaphragmatic hernia lamb model. Crit Care Med 23: 1858–1863
Murray IP, Modell JH, Gallagher TJ, Banner MJ 1984 Titration of PEEP by the arterial minus end-tidal carbon dioxide gradient. Chest 85: 100–104
Mates EA, Jackson JC, Hildebrandt J, Truog WE, Standaert TA, Hlastala MP 1994 Respiratory gas exchange and inert gas retention during partial liquid ventilation. In: Hogen MC, Mathieu-Costello O, Poole DC, Wagner PD (eds) Oxygen Transport to Tissue XVI. Plenum, New York, 427–435
Moomey CB, Fabian TC, Croce MA, Melton SM, Proctor KG 1998 Cardiopulmonary function after pulmonary contusion partial liquid ventilation. J Trauma 45: 283–290
Fujino Y, Godon S, Chiche JD, Hromi J, Kacmarek M 2000 Partial liquid ventilation ventilates better than gas ventilation. Am J Respir Crit Care Med 161: 650–657
Nunn JF 1993 Nunn's Applied Respiratory Physiology. Butterworth-Heinemann Ltd, Oxford, 171–178
Mates EA, Tarczy-Hornoch P, Hildebrandt J, Jackson JC, Hlastala MP 1996 Negative slope of exhaled CO2 profile: implications for ventilation heterogeneity during partial liquid ventilation. In: Ince C, Kesecloglu J, Telci L, Akpir K (eds) Oxygen Transport to Tissue XVII. Plenum, New York, 585–597
Mates EA, Hildebrandt J, Jackson JC, Tarczy-Hornoch P, Hlastala MP 1997 Shunt ventilation-perfusion distribution during partial liquid ventilation in healthy piglets. J Appl Physiol 82: 933–942
Breen PH, Mazumdar B, Skinner SC 1996 Comparison of end-tidal PCO2 average alveolar expired PCO2 during positive end-expiratory pressure. Anesth Analg 82: 368–373
Kaisers U, Max M, Schnabel R, Böhm S, Hendrik ER, Rossaint R, Lachmann B 1996 Partial liquid ventilation with FC 3280 in experimental lung injury: dose-dependent improvement of gas exchange lung mechanics. Appl Cardiopulm Pathophysiol 6: 163–170
Nesti FD, Fuhrmann BP, Papo MC, Steinhorn DM, Hernan LJ, Leach CL, Holm B, Paczan P, Burak B 1994 Perfluorocarbon-associated gas exchange in gastric aspiration. Crit Care Med 22: 1445–1452
Curtis SE, Fuhrmann BP, Howland DF, DeFrancisis M, Motoyama EK 1991 Cardiac output during liquid breathing in newborn piglets. Crit Care Med 19: 225–230
Wolfson MR, Greenspan JS, Deoras KS, Rubinstein SD, Shaffer TH 1992 Comparison of gas liquid ventilation: clinical, physiological, histological correlates. J Appl Physiol 72: 1024–1031
Shaffer TH, Douglas PR, Lowe CA, Bhutani VK 1983 The effects of liquid ventilation on cardiopulmonary function in preterm lambs. Pediatr Res 17: 303–306
Houmes RJM, Verbrugge SJC, Hendrik ER, Lachmann B 1995 Hemodynamic effects of partial liquid ventilation with perfluorocarbon in acute lung injury. Intensive Care Med 21: 966–972
Acknowledgements
The authors want to thank Mrs. Gabriele Gruber, Mrs. Elisabeth Perstaller, Mrs. Ursula Schreiner, Mrs. Franziska Sommer, and Mrs. Simone Sprinz for their great support throughout the experiment and Mr. Peter Rehak, PhD, for statistical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rödl, S., Urlesberger, B., Knez, I. et al. Partial Liquid Ventilation Versus Conventional Mechanical Ventilation with High PEEP and Moderate Tidal Volume in Acute Respiratory Failure in Piglets. Pediatr Res 52, 225–232 (2002). https://doi.org/10.1203/00006450-200208000-00015
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200208000-00015